RESEARCH PAPER
Occurrence of Toxoplasma gondii infection among free-living small rodents and insectivores in the Lublin Province – the role of these animals in epidemiology of toxoplasmosis
1
Instytut Medycyny Wsi, Lublin, Polska
Corresponding author
Med Og Nauk Zdr. 2021;27(4):448-452
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
Free-living small rodents and insectivores are an important part of the diet of many species of carnivores and omnivores, contributing to the spread of T. gondii infections in the environment. The aim of the study was to assess the occurrence of T. gondii infection in the free-living small mammals population from the Lublin Province, and to determine their potential importance in spreading this invasion in the environment. The research was based on the detection and analysis of the parasite DNA isolated from tissue samples.
Material and methods:
Sixty dead, small mammals from the Lublin Province, belonging to 7 species were collected. DNA from animal tissue samples was isolated using a commercial kit (QIAGEN). Tests for the presence of T. gondii DNA were performed by nested and Real-time PCR based on the amplification of the B1 fragment gene. To determine the clonal type of the parasite, selected DNA isolates were tested by RFLP PCR with 12 genetic markers. The amplification products of selected samples were subjected to sequencing and phylogenetic analysis.
Results:
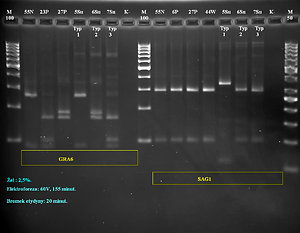
Overall, among 180 tissue samples from 60 small mammals tested in nested and/or Real time PCR, T. gondii DNA was found in samples from 10 rodents (16.7%). RFLP-PCR and sequence analysis revealed the presence of T. gondii clonal types II and III in the majority of the tested samples.
Conclusions:
The results of study indicate a high degree of T. gondii infection in free-living small mammals (especially rodents) from the Lublin Province (16.7%), and confirm the important role of these animals as a reservoir and vector of the parasite in the environment.
Free-living small rodents and insectivores are an important part of the diet of many species of carnivores and omnivores, contributing to the spread of T. gondii infections in the environment. The aim of the study was to assess the occurrence of T. gondii infection in the free-living small mammals population from the Lublin Province, and to determine their potential importance in spreading this invasion in the environment. The research was based on the detection and analysis of the parasite DNA isolated from tissue samples.
Material and methods:
Sixty dead, small mammals from the Lublin Province, belonging to 7 species were collected. DNA from animal tissue samples was isolated using a commercial kit (QIAGEN). Tests for the presence of T. gondii DNA were performed by nested and Real-time PCR based on the amplification of the B1 fragment gene. To determine the clonal type of the parasite, selected DNA isolates were tested by RFLP PCR with 12 genetic markers. The amplification products of selected samples were subjected to sequencing and phylogenetic analysis.
Results:
Overall, among 180 tissue samples from 60 small mammals tested in nested and/or Real time PCR, T. gondii DNA was found in samples from 10 rodents (16.7%). RFLP-PCR and sequence analysis revealed the presence of T. gondii clonal types II and III in the majority of the tested samples.
Conclusions:
The results of study indicate a high degree of T. gondii infection in free-living small mammals (especially rodents) from the Lublin Province (16.7%), and confirm the important role of these animals as a reservoir and vector of the parasite in the environment.
REFERENCES (30)
1.
Hejlíček K, Literák I, Nezval J. Toxoplasmosis in wild mammals from the Czech Republic. J Wildelife Dis. 1997; 33: 480–485.
2.
Kijlstra A, Meerburg B, Cornelissen J, et al. The role of rodents and shrews in the transmission of Toxoplasma gondii to pigs. Vet Parasitol. 2008; 56: 183–190.
3.
Waindok P, Özbakıs-Beceriklisoy G, Janecek-Erfurth E, et al. Parasites in brains of wild rodents (Arvicolinae and Murinae) in the city of Leipzig, Germany. Int J Parasitol Parasites Wildl. 2019; 10: 211–217.
4.
Sroka J, Karamon J, Wójcik-Fatla A, et al. Toxoplasma gondii infection in slaughtered pigs and cattle in Poland: seroprevalence, molecular detection and characterization of parasites in meat. Parasites Vectors. 2020; 23: 223. https://doi.org/10.1186/s13071....
5.
Kijlstra A, Jongert E. Control of the risk of human toxoplasmosis transmitted by meat. Int J Parasitol. 2008; 38: 1359–1370.
6.
Shwab EK, Zhu XQ, Majumdar D, et al. Geographical patterns of Toxoplasma gondii genetic diversity revealed by multilocus PCR-RFLP genotyping. Parasitology. 2014; 141(4): 453–461. doi.org/10.1017/S0031182013001844.
7.
de Sousa S, Ajzenberg D, Canada N, et al. Biologic and molecular characterization of Toxoplasma gondii isolates from pigs from Portugal. Vet Parasitol. 2006; 135: 133–136.
8.
Turčeková L, Antolová D, Reiterová K, et al. Occurrence and genetic characterization of Toxoplasma gondii in naturally infected pigs. Acta Parasitol. 2013; 58: 361–366.
9.
Khan A, Dubey JP, Su C, et al. Genetic analyses of atypical Toxoplasma gondii strains reveal a fourth clonal lineage in North America. Int J Parasitol. 2011; 41: 645–55.
10.
Karakavuk M, Aldemir D, Mercier A, et al. Prevalence of toxoplasmosis and genetic characterization of Toxoplasma gondii strains isolated in wild birds of prey and their relation with previously isolated strains from Turkey. PLoS ONE. 2018; 13(4): e0196159. https://doi.org/10.1371/journa....
11.
Grigg ME, Boothroyd JC. Rapid identification of virulent type I strains of the protozoan pathogen Toxoplasma gondii by PCR restriction fragment length polymorphism analysis at the B1 gene. J Clin Microbiol. 2001; 39: 398–400.
12.
Lin MH, Chen TC, Kuo TT, et al. Real-time PCR for quantitative detection of Toxoplasma gondii. J Clin Microbiol. 2000; 38: 4121–4125.
13.
Su C, Shwab EK, Zhou P, et al. Moving towards an integrated approach to molecular detection and identification of Toxoplasma gondii. Parasitology. 2010; 137: 1–11.
14.
Meerburg BG, Singleton GR, Kijlstra A. Rodent-borne diseases and their risks for public health. Crit Rev Microbiol 2009; 35: 221–70. doi: 10.1080/10408410902989837.
15.
Meerburg BG. Rodents are a risk factor for the spreading of pathogens on farms. Vet Microbiol. 2010; 142: 464–465.
16.
Galeh TM, Sarvi S, Montazeri M, et al. Global status of Toxoplasma gondii seroprevalence in rodents: A Systematic Review and Meta-Analysis. Front Vet Sci. 2020; 7: 461.
17.
Reiterová K, Antolová D, Zalesny G, et al. Small rodents—Permanent reservoirs of toxocarosis in different habitats of Slovakia. Helminthologia. 2013; 50: 20–26.
18.
Grzybek M, Antolová D, Tołkacz K, et al. Seroprevalence of Toxoplasma gondii among sylvatic rodents in Poland. Animals. 2021; 11: 1048. https://doi.org/10.3390/ani110....
19.
Meerburg BG, De Craeye S, Dierick K, et al. Neospora caninum and Toxoplasma gondii in brain tissue of feral rodents and insectivores caught on farms in the Netherlands. Vet Parasitol. 2012; 184: 317–20.
20.
Gotteland C, Chaval Y, Villena I, et al. Species or local environment, what determines the infection of rodents by Toxoplasma gondii? Parasitology. 2014; 141: 259–68. doi: 10.1017/S0031182013001522.
21.
Afonso E, Poulle ML, Lemoine M. et al. Prevalence of Toxoplasma gondii in small mammals from the Ardennes region, France. Folia Parasitol. 2007; 54: 313–314.
22.
Araujo JB, da Silva AV, Rosa RC, et al. Isolation and multilocus genotyping of Toxoplasma gondii in seronegative rodents in Brazil. Vet Parasitol. 2010; 174: 328–331.
23.
Khademvatan S, Foroutan M, Hazrati-Tappeh K, et al. Toxoplasmosis in rodents: a systematic review and meta-analysis in Iran (2017). J Infect Public Heal. 2017; 10: 487–493. doi: 10.1016/j.jiph.2017.01.021.
24.
Turčeková L, Hurníková Z, Spišák F, et al. Toxoplasma gondii in protected wildlife in the Tatra National Park (TANAP), Slovakia. Ann Agric Environ Med. 2014; 21(2): 235–238. doi: 10.5604/1232-1966.1108582.
25.
Vujanic M, Ivovic V, Kataranovski M, et al. Toxoplasmosis in naturally infected rodents in Belgrade, Serbia. Vector Borne Zoonotic Dis. 2011; 11: 1209–1211.
26.
Sroka J, Karamon J, Wójcik-Fatla A, et al. Toxoplasma gondii infection in selected species of free-living animals in Poland. Ann Agric Environ Med. 2019; 26(4): 656–660. doi: 10.26444/aaem/114930.
27.
Marshall PA, Hughes JM, Williams RH, et al. Detection of high levels of congenital transmission of Toxoplasma gondii in natural urban populations of Mus domesticus. Parasitology 2004; 128: 39–42.
28.
Hughes JM, Williams RH, Morley EK, et al. The prevalence of Neospora caninum and co-infection with Toxoplasma gondii by PCR analysis in naturally occurring mammal populations. Parasitology 2006; 132: 29–36.
29.
Murphy RG, Williams RH, Hughes JM, et al. The urban house mouse (Mus domesticus) as a reservoir of infection for the human parasite Toxoplasma gondii: an unrecognised public health issue? Int J Environ Health Res. 2008; 18: 177–185.
30.
Owen MR, Trees AJ. Vertical transmission of Toxoplasma gondii from chronically infected house (Mus musculus) and field ( Apodemus sylvaticus) mice determined by polymerase chain reaction. Parasitology 1998; 116: 299–304.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.