REVIEW PAPER
Problems of carriage of CPE strains – epidemiology, review of eradication methods and risk of transformation into infection
1
Oddział Chorób Wewnętrznych, Endokrynologii i Diabetologii, Wojewódzki Szpital Specjalistyczny im. Stefana Kardynała Wyszyńskiego
Corresponding author
Barbara Szostak
Oddział Chorób Wewnętrznych, Endokrynologii i Diabetologii, Wojewódzki Szpital Specjalistyczny im. Stefana Kardynała Wyszyńskiego
Oddział Chorób Wewnętrznych, Endokrynologii i Diabetologii, Wojewódzki Szpital Specjalistyczny im. Stefana Kardynała Wyszyńskiego
Med Og Nauk Zdr. 2024;30(4):271-277
KEYWORDS
eradicationcarriageC Adres do korespondencji: Barbara SzostakOddział Chorób WewnętrznychPEantibiotic resistance
TOPICS
ABSTRACT
Introduction and objective:
Carbapenemase-producing Enterobacterales are the most dangerous strains from the aspect of epidemiology, characterized by their ease of spread and ability for long-term colonization. The aim of this article is to characterize the phenomenon of CPE carriage, and present the latest methods of its eradication. The article also describes the risk of transformation of carriage into infection with multidrug-resistant gram-negative bacilli
Review methods:
A review of articles on antibiotic resistance of Carbapenemase-producing Enterobacterales was carried out in PubMed and Google Scholar database. The search terms used were: Carbapenemase-producing Enterobacteriaceae, CPE eradication, carriage; recommendations of Polish and foreign scientific societies.
Brief description of the state of knowledge:
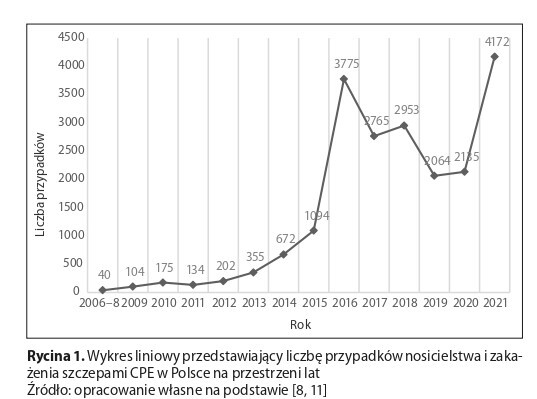
Over the years, in Poland, an increase has been observed in the number of outbreaks caused by CPE strains. Among the most important factors increasing the risk of colonization with these multidrugresistant bacteria is early hospitalization, especially in the ICU. There are many factors determining the duration of CPE carriage. According to the studies reviewed, most patients spontaneously decolonize within a year. In the literature, methods of CPE eradication include SDD, FTM, and probiotics. In the absence of reliable studies on the above methods, Polish and foreign recommendations do not recommend their routine use.
Summary:
The cited information is a confirmation of the risk of post-antibiotic era. Intensification of work on methods to prevent the spread of multidrug-resistant bacteria and reliable studies on methods of CPE eradication are required
Carbapenemase-producing Enterobacterales are the most dangerous strains from the aspect of epidemiology, characterized by their ease of spread and ability for long-term colonization. The aim of this article is to characterize the phenomenon of CPE carriage, and present the latest methods of its eradication. The article also describes the risk of transformation of carriage into infection with multidrug-resistant gram-negative bacilli
Review methods:
A review of articles on antibiotic resistance of Carbapenemase-producing Enterobacterales was carried out in PubMed and Google Scholar database. The search terms used were: Carbapenemase-producing Enterobacteriaceae, CPE eradication, carriage; recommendations of Polish and foreign scientific societies.
Brief description of the state of knowledge:
Over the years, in Poland, an increase has been observed in the number of outbreaks caused by CPE strains. Among the most important factors increasing the risk of colonization with these multidrugresistant bacteria is early hospitalization, especially in the ICU. There are many factors determining the duration of CPE carriage. According to the studies reviewed, most patients spontaneously decolonize within a year. In the literature, methods of CPE eradication include SDD, FTM, and probiotics. In the absence of reliable studies on the above methods, Polish and foreign recommendations do not recommend their routine use.
Summary:
The cited information is a confirmation of the risk of post-antibiotic era. Intensification of work on methods to prevent the spread of multidrug-resistant bacteria and reliable studies on methods of CPE eradication are required
REFERENCES (50)
1.
World Health Organization. 10 global health issues to track in 2021. https://www.who.int/news-room/... (access 2024.05.22).
2.
Murray CJL, Shunji Ikuta K, Sharara F, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022;399(10325): 629–55. https://doi.org/10.1016/S0140-....
3.
Dzierżanowska D, Bucki R, Durnaś B, et al. Antybiotykoterapia praktyczna Wydanie 6 α-medica press; 2018. p. 49–67.
4.
Hryniewicz W, Kuch A, Wanke-Rytt M, et al. Pałeczki Enterobacterales wytwarzające karbapenemazy (CPE) Epidemiologia, diagnostyka, leczenie i profilaktyka zakażeń. Wydanie pierwsze. Warszawa: Narodowy Instytut Leków; 2022.
5.
Cassini A, Högberg LD, Plachouras D, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19(1):56–66. doi:10.1016S1473-3099(18)30605-4.
6.
Ma J, Song X, Li M, et al. Global spread of carbapenem-resistant Enterobacteriaceae: Epidemiological features, resistance mechanisms, detection and therapy. Microbiol Res. 2023;266:127249. doi:10.1016j.micres.2022.127249.
7.
Wang Q, Wang X, Wang J, et al. Phenotypic and Genotypic Characterization of Carbapenem-resistant Enterobacteriaceae: Data From a Longitudinal Large-scale CRE Study in China (2012–2016). Clin Infect Dis. 2018;67(suppl_2):S196 S205. doi:10.1093/cid/ciy660.
8.
Główny Inspektorat Sanitarny. Stan Sanitarny Kraju w 2022 roku. Warszawa: Główny Inspektorat Sanitarny; lipiec 2023.
9.
Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11(4):589–603. doi:10.1128/CMR.11.4.589.
10.
Brink AJ. Epidemiology of carbapenem-resistant Gram-negative infections globally. Curr Opin Infect Dis. 2019;32(6):609–616. doi:10.1097/QCO.0000000000000608.
11.
Literacka E, Żabicka D, Hryniewicz W, et al. Dane Krajowego Ośrodka Referencyjnego ds. Lekowrażliwości Drobnoustrojów (KORLD), dotyczące pałeczek Enterobacterales wytwarzających karbapenemazy NDM, KPC, VIM i OXA-48 na terenie Polski w latach 2006 – 2018. Warszawa: Narodowy Instytut Leków; 2019.
12.
European Centre for Disease Prevention and Control. Antimicrobial resistance in the EU/EEA (EARS-Net) – Annual Epidemiological Report 2022. Stockholm: ECDC; 2023.
13.
Tuhamize B, Bazira J. Carbapenem-resistant Enterobacteriaceae in the livestock, humans and environmental samples around the globe: a systematic review and meta-analysis. Sci Rep. 2024;14(1):16333. Published 2024 Jul 15. doi:10.1038/s41598-024-64992-8.
14.
Walsh TR, Weeks J, Livermore DM, Toleman MA. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis. 2011;11(5):355–362. doi:10.1016/S1473-3099(11)70059-7.
15.
Gu C, Li X, Zou H, et al. Clonal and plasmid-mediated dissemination of environmental carbapenem-resistant Enterobacteriaceae in large animal breeding areas in northern China. Environ Pollut. 2022;297:118800. doi:10.1016/j.envpol.2022.118800.
16.
Colosi IA, Baciu AM, Opriș RV, et al. Prevalence of ESBL, AmpC and Carbapenemase-Producing Enterobacterales Isolated from Raw Vegetables Retailed in Romania. Foods. 2020;9(12):1726. Published 2020 Nov 24. doi:10.3390/foods9121726.
17.
Zurfluh K, Poirel L, Nordmann P, et al. First detection of Klebsiella variicola producing OXA-181 carbapenemase in fresh vegetable imported from Asia to Switzerland. Antimicrob Resist Infect Control. 2015;4:38. Published 2015 Oct 6. doi:10.1186/s13756-015-0080-5.
18.
Lin L, Ke ZY, Wang Y, et al. Efficacy of preoperative screening and decolonization for staphylococcus aureus in total joint arthroplasty: A meta-analysis. Asian J Surg. 2021 Jun;44(6):807–818. https://doi.org/10.1016/j.asjs....
19.
van Loon K, Voor In ,t Holt AF, Vos MC. A Systematic Review and Meta-analyses of the Clinical Epidemiology of Carbapenem-Resistant Enterobacteriaceae. Antimicrob Agents Chemother. 2017 Dec 21;62(1):e01730–17. https://doi.org/10.1128/AAC.01....
20.
Zhu L, Liang L, Hui J, et al. Relationship between antibiotic exposure and carbapenem-resistant Klebsiella pneumoniae infection within four types of control patients: A systematic review and meta-analysis. J Glob Antimicrob Resist. 2023 Jun;33:137. 151. https://doi.org/10.1016/j.jgar....
21.
Magiorakos A, P, Struelens M, Jasir A, et al. Risk assessment on the spread of carbapenemase-producing Enterobacteriaceae (CPE). Stockholm: European Centre for Disease Prevention and Control (ECDC); 2011.
22.
Yang P, Chen Y, Jiang S, et al. Association between antibiotic consumption and the rate of carbapenem-resistant Gram-negative bacteria from China based on 153 tertiary hospitals data in 2014. Antimicrob Resist Infect Control. 2018 Nov 19;7:137. https://doi.org/10.1186/s13756....
23.
Haverkate MR, Weiner S, Lolans K, et al. Duration of Colonization With Klebsiella pneumoniae Carbapenemase-Producing Bacteria at Long-Term Acute Care Hospitals in Chicago, Illinois. Open Forum Infect Dis. 2016 Aug 30;3(4):ofw178. https://doi.org/10.1093/ofid/o....
24.
Mo Y, Hernandez-Koutoucheva A, Musicha P, et al. Duration of Carbapenemase-Producing Enterobacteriaceae Carriage in Hospital Patients. Emerg Infect Dis. 2020 Sep;26(9):2182–2185. doi:10.3201/eid2609.190592.
25.
Marimuthu K, Mo Y, Ling ML, et al. Household transmission of carbapenemase-producing Enterobacteriaceae: a prospective cohort study. J Antimicrob Chemother. 2021 Apr 13;76(5):1299–1302. doi:10.1093/jac/dkaa561.
26.
Haverkate MR, Platteel TN, Fluit AC, et al. Quantifying within-household transmission of extended-spectrum β-lactamase-producing bacteria. Clin Microbiol Infect. 2017 Jan;23(1):46.e1-46.e7. https://doi.org/10.1016/j.cmi.....
27.
Centers for Disease Control and Prevention. Carbapenem-resistant Enterobacterales (CRE) Infection Control. https://www.cdc.gov/cre/hcp/in... (access 2024.05.22).
28.
French CE, Coope C, Conway L, et al. Control of carbapenemase-producing Enterobacteriaceae outbreaks in acute settings: an evidence review. J Hosp Infect. 2017 Jan;95(1):3–45. https://doi.org/10.1016/j.jhin....
29.
Kochar S, Sheard T, Sharma R, et al. Success of an infection control program to reduce the spread of carbapenem-resistant Klebsiella pneumoniae. Infect Control Hosp Epidemiol. 2009;30(5):447–452. doi:10.1086/596734.
30.
Enfield K, Huq N, Gosseling M, et al. Control of simultaneous outbreaks of carbapenemase-producing enterobacteriaceae and extensively drug-resistant Acinetobacter baumannii infection in an intensive care unit using interventions promoted in the Centers for Disease Control and Prevention 2012 carbapenemase-resistant Enterobacteriaceae Toolkit. Infect Control Hosp Epidemiol. 2014;35(7):810–817. doi:10.1086/676857.
31.
Weintrob AC, Roediger MP, Barber M, et al. Natural history of colonization with gram-negative multidrug-resistant organisms among hospitalized patients. Infect Control Hosp Epidemiol. 2010 Apr;31(4):330–7. https://doi.org/10.1086/651304.
32.
Seong H, Lee SK, Cheon J, et al. Fecal Microbiota Transplantation for multidrug-resistant organism: Efficacy and Response prediction. J Infect. 2020 Nov;81(5):719–725. https://doi.org/10.1016/j.jinf....
33.
Tavoukjian V. Faecal microbiota transplantation for the decolonization of antibiotic-resistant bacteria in the gut: a systematic review and meta-analysis. J Hosp Infect. 2019 Jun;102(2):174–188. https://doi.org/10.1016/j.jhin....
34.
Bar-Yoseph H, Carasso S, Shklar S, et al. Oral Capsulized Fecal Microbiota Transplantation for Eradication of Carbapenemase-producing Enterobacteriaceae Colonization With a Metagenomic Perspective. Clin Infect Dis. 2021 Jul 1;73(1):e166-e175. https://doi.org/10.1093/cid/ci....
35.
Mascolo A, Carannante N, Mauro GD, et al. Decolonization of drug-resistant Enterobacteriaceae carriers: A scoping review of the literature. J Infect Public Health. 2023 Mar;16(3):376–383. doi:10.1016/j.jiph.2023.01.009.
36.
Bar-Yoseph H, Lulu C, Shklar S, et al. Efficacy of a hospital policy of selective digestive decontamination for carbapenem-resistant Enterobacterales carriers: prospective before-after study. J Hosp Infect. 2020 Nov;106(3):495–499. https://doi.org/10.1016/j.jhin....
37.
Myburgh JA, Seppelt IM, Goodman F, et al. Effect of Selective Decontamination of the Digestive Tract on Hospital Mortality in Critically Ill Patients Receiving Mechanical Ventilation: A Randomized Clinical Trial. JAMA. 2022 Nov 15;328(19):1911–1921. https://doi.org/10.1001/jama.2....
38.
Fariñas MC, González-Rico C, Fernández-Martínez M, et al. Oral decontamination with colistin plus neomycin in solid organ transplant recipients colonized by multidrug-resistant Enterobacterales: a multicentre, randomized, controlled, open-label, parallel-group clinical trial. Clin Microbiol Infect. 2021 Jun;27(6):856–863. https://doi.org/10.1016/j.cmi.....
39.
Hill C, Guarner F, Reid G, Gibson GR, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014 Aug;11(8):506–14. https://doi.org/10.1038/nrgast....
40.
Wieërs G, Verbelen V, Van Den Driessche M, et al. Do Probiotics During In-Hospital Antibiotic Treatment Prevent Colonization of Gut Microbiota With Multi-Drug-Resistant Bacteria? A Randomized Placebo-Controlled Trial Comparing Saccharomyces to a Mixture of Lactobacillus, Bifidobacterium, and Saccharomyces. Front Public Health. 2021 Mar 8;8:578089. https://doi.org/10.3389/fpubh.....
41.
Tacconelli E, Mazzaferri F, de Smet AM, et al. ESCMID-EUCIC clinical guidelines on decolonization of multidrug-resistant Gram-negative bacteria carriers. Clin Microbiol Infect. 2019 Jul;25(7):807–817. https://doi.org/10.1016/j.cmi.....
42.
Pérez-Nadales E, Cano Á, Recio M, et al. Randomised, double-blind, placebo-controlled, phase 2, superiority trial to demonstrate the effectiveness of faecal microbiota transplantation for selective intestinal decolonisation of patients colonised by carbapenemase-producing Klebsiella pneumoniae (KAPEDIS). BMJ Open. 2022 Apr 6;12(4):e058124. https://doi.org/10.1136/bmjope....
43.
Lin MY, Lyles-Banks RD, Lolans K, et al. Centers for Disease Control and Prevention Epicenters Program. The importance of long-term acute care hospitals in the regional epidemiology of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Clin Infect Dis. 2013 Nov;57(9):1246–52. https://doi.org/10.1093/cid/ci....
44.
Adelman MW, Woodworth MH, Langelier C, et al. The gut microbiome›s role in the development, maintenance, and outcomes of sepsis. Crit Care. 2020 Jun 1;24(1):278. https://doi.org/10.1186/s13054....
45.
Kogut MH, Lee A, Santin E. Microbiome and pathogen interaction with the immune system. Poult Sci. 2020 Apr;99(4):1906–1913. https://doi.org/10.1016/j.psj.....
47.
Gutiérrez-Gutiérrez B, Salamanca E, de Cueto M, et al. Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect Dis. 2017 Jul;17(7):726–734. https://doi.org/10.1016/S1473-....
48.
van der Zwaluw K, de Haan A, Pluister GN, et al. The carbapenem inactivation method (CIM), a simple and low-cost alternative for the Carba NP test to assess phenotypic carbapenemase activity in gram-negative rods. PLoS One. 2015 Mar 23;10(3):e0123690. https://doi.org/10.1371/journa....
49.
Baeza LL, Pfennigwerth N, Greissl C, et al. Comparison of five methods for detection of carbapenemases in Enterobacterales with proposal of a new algorithm. Clin Microbiol Infect. 2019;25(10):1286.e9–1286.e15. doi:10.1016/j.cmi.2019.03.003.
50.
UK Standards for Microbiology Investigations Detection of bacteria with carbapenem-hydrolysing β-lactamases (carbapenemases). Public Health England (PHE), 2022.
Share
RELATED ARTICLE