Online first
Bieżący numer
Archiwum
O czasopiśmie
Polityka etyki publikacyjnej
System antyplagiatowy
Instrukcje dla Autorów
Instrukcje dla Recenzentów
Rada Redakcyjna
Komitet Redakcyjny
Recenzenci
Wszyscy recenzenci
2024
2023
2022
2021
2020
2019
2018
2017
2016
Kontakt
Bazy indeksacyjne
Klauzula przetwarzania danych osobowych (RODO)
PRACA PRZEGLĄDOWA
Postcovidowy zespół jelita nadwrażliwego – znaczenie dietoterapii oraz probiotykoterapii
1
Studenckie Koło Naukowe Żywienia Człowieka i Dietetyki, Wydział Technologii Żywności, Uniwersytet Rolniczy im. H. Kołłątaja w Krakowie, Polska
2
Katedra Żywienia Człowieka i Dietetyki, Wydział Technologii Żywności, Uniwersytet Rolniczy im. H. Kołłątaja w Krakowie, Polska
Autor do korespondencji
Dominika Grońska
Studenckie Koło Naukowe Żywienia Człowieka i Dietetyki, Wydział Technologii Żywności, Uniwersytet Rolniczy im. H. Kołłątaja w Krakowie, al. Mickiewicza 21, 31-120, Kraków, Polska
Studenckie Koło Naukowe Żywienia Człowieka i Dietetyki, Wydział Technologii Żywności, Uniwersytet Rolniczy im. H. Kołłątaja w Krakowie, al. Mickiewicza 21, 31-120, Kraków, Polska
Med Og Nauk Zdr. 2022;28(4):295-300
SŁOWA KLUCZOWE
DZIEDZINY
STRESZCZENIE
Wprowadzenie i cel:
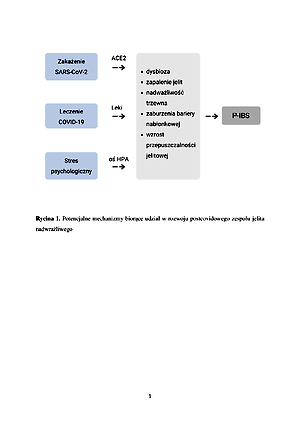
Aktualnym wyzwaniem dla medycyny i dziedzin pokrewnych staje się opracowanie metod i algorytmów postępowania w przypadku rozwoju nowych chorób, do których niewątpliwie należy postcovidowy zespół jelita nadwrażliwego (P-IBS). Niniejszy przegląd ma na celu przedstawienie aktualnych dowodów naukowych dotyczących wpływu choroby koronawirusowej 2019 na rozwój postcovidowego zespołu jelita nadwrażliwego.
Metody przeglądu:
Przeglądu piśmiennictwa dokonano, wykorzystując dane pochodzące z wyszukiwarek PubMed, Google Scholar oraz bazy danych Medline.
Opis stanu wiedzy:
Obecnie nie istnieją żadne wytyczne czy rekomendacje dotyczące postępowania terapeutycznego u pacjentów z postcovidowym zespołem jelita nadwrażliwego. Stąd też dokonano przeglądu ogólnego standardu leczenia pacjentów z zespołem jelita nadwrażliwego (IBS). Uznaje się, że dobrym wsparciem w terapii IBS może być zastosowanie spersonalizowanej diety low-FODMAP pod kontrolą dietetyka klinicznego. Ponadto celowe wydaje się wdrożenie personalizowanej terapii probiotycznej opartej na suplementacji szczepów o udowodnionym działaniu klinicznym. Duże nadzieje rodzi również nowa gałąź probiotykoterapii, oparta na zastosowaniu psychobiotyków, jako forma wsparcia terapii pacjentów z IBS.
Podsumowanie:
Zastosowanie u pacjentów spersonalizowanej diety low-FODMAP oraz celowanej probiotykoterapii, a w tym psychobiotykoterapii, może stanowić skuteczną metodę wsparcia leczenia pacjentów cierpiących na postcovidowy zespół jelita nadwrażliwego. Aktualnie nie istnieją żadne wytyczne dotyczące rutynowego stosowania wymienionych metod. Wskazuje się na potrzebę prowadzenia dalszych badań naukowych nad mechanizmami działania i skuteczności interwencji w kontekście P-IBS
Aktualnym wyzwaniem dla medycyny i dziedzin pokrewnych staje się opracowanie metod i algorytmów postępowania w przypadku rozwoju nowych chorób, do których niewątpliwie należy postcovidowy zespół jelita nadwrażliwego (P-IBS). Niniejszy przegląd ma na celu przedstawienie aktualnych dowodów naukowych dotyczących wpływu choroby koronawirusowej 2019 na rozwój postcovidowego zespołu jelita nadwrażliwego.
Metody przeglądu:
Przeglądu piśmiennictwa dokonano, wykorzystując dane pochodzące z wyszukiwarek PubMed, Google Scholar oraz bazy danych Medline.
Opis stanu wiedzy:
Obecnie nie istnieją żadne wytyczne czy rekomendacje dotyczące postępowania terapeutycznego u pacjentów z postcovidowym zespołem jelita nadwrażliwego. Stąd też dokonano przeglądu ogólnego standardu leczenia pacjentów z zespołem jelita nadwrażliwego (IBS). Uznaje się, że dobrym wsparciem w terapii IBS może być zastosowanie spersonalizowanej diety low-FODMAP pod kontrolą dietetyka klinicznego. Ponadto celowe wydaje się wdrożenie personalizowanej terapii probiotycznej opartej na suplementacji szczepów o udowodnionym działaniu klinicznym. Duże nadzieje rodzi również nowa gałąź probiotykoterapii, oparta na zastosowaniu psychobiotyków, jako forma wsparcia terapii pacjentów z IBS.
Podsumowanie:
Zastosowanie u pacjentów spersonalizowanej diety low-FODMAP oraz celowanej probiotykoterapii, a w tym psychobiotykoterapii, może stanowić skuteczną metodę wsparcia leczenia pacjentów cierpiących na postcovidowy zespół jelita nadwrażliwego. Aktualnie nie istnieją żadne wytyczne dotyczące rutynowego stosowania wymienionych metod. Wskazuje się na potrzebę prowadzenia dalszych badań naukowych nad mechanizmami działania i skuteczności interwencji w kontekście P-IBS
Introduction and objective:
The current challenge for medicine and the related disciplines is the development of methods and algorithms for dealing with the development of new diseases which undoubtedly include post-Covid-19 irritable bowel syndrome (P-IBS). This review is aimed at presentation of up-to-date scientific evidence on the impact of COVID-19 disease on development of post-Covid-19 irritable bowel syndrome.
Review methods:
The literature was reviewed using search engine data from PubMed, Google Scholar and the Medline databases.
Abbreviated description of the state of knowledge:
At present, there are no guidelines or recommendations concerning therapeutic management of patients with post-Covid-19 irritable bowel syndrome. Therefore, the general standard of treatment for patients with irritable bowel syndrome (IBS) was reviewed. It is considered that the use of personalized low-FODMAP diet under the control of a clinical dietitian may be a good support in the treatment of IBS. Moreover, it seems advisable to supply personalized probiotic therapy based on the supplementation of strains with proven clinical effect. A new branch of probiotic therapy based on the also brings high hopes.
Summary:
The use of personalized low-FODMAP dietary supply and targeted probiotic therapy, including psychobiotherapy, could potentially be an effective method of supporting the treatment of patients suffering from post-Covid-19 irritable bowel syndrome. Currently, there are no guidelines for the routine use of the aforementioned methods. The need for further scientific research into the mechanisms of action and effectiveness of interventions in the context of P-IBS is indicated.
The current challenge for medicine and the related disciplines is the development of methods and algorithms for dealing with the development of new diseases which undoubtedly include post-Covid-19 irritable bowel syndrome (P-IBS). This review is aimed at presentation of up-to-date scientific evidence on the impact of COVID-19 disease on development of post-Covid-19 irritable bowel syndrome.
Review methods:
The literature was reviewed using search engine data from PubMed, Google Scholar and the Medline databases.
Abbreviated description of the state of knowledge:
At present, there are no guidelines or recommendations concerning therapeutic management of patients with post-Covid-19 irritable bowel syndrome. Therefore, the general standard of treatment for patients with irritable bowel syndrome (IBS) was reviewed. It is considered that the use of personalized low-FODMAP diet under the control of a clinical dietitian may be a good support in the treatment of IBS. Moreover, it seems advisable to supply personalized probiotic therapy based on the supplementation of strains with proven clinical effect. A new branch of probiotic therapy based on the also brings high hopes.
Summary:
The use of personalized low-FODMAP dietary supply and targeted probiotic therapy, including psychobiotherapy, could potentially be an effective method of supporting the treatment of patients suffering from post-Covid-19 irritable bowel syndrome. Currently, there are no guidelines for the routine use of the aforementioned methods. The need for further scientific research into the mechanisms of action and effectiveness of interventions in the context of P-IBS is indicated.
Grońska D, Drzewowska K. Postcovidowy zespół jelita nadwrażliwego – znaczenie dietoterapii oraz probiotykoterapii. Med Og Nauk Zdr. 2022; 28(4): 295–300. doi: 10.26444/monz/155260
REFERENCJE (73)
1.
Settanni CR, Ianiro G, Ponziani FR, et al. COVID-19 as a trigger
of irritable bowel syndrome: A review of potential mechanisms. World J Gastroenterol. 2021; 27(43): 7433–7445. doi:10.3748/wjg.v27.i43.7433
2.
Parasher A. COVID-19: Current understanding of its Pathophysiology, Clinical presentation and Treatment. Postgrad Med J. 2021; 97(1147): 312–320. doi:10.1136/postgradmedj-2020-13857710.
3.
Cao TT, Zhang GQ, Pellegrini E, et al. COVID-19 and its effects on the digestive system. World J Gastroenterol. 2021; 27(24): 3502–3515. doi:10.3748/wjg.v27.i24.3502.
4.
Ma C, Cong Y, Zhang H. COVID-19 and the Digestive System. Am J Gastroenterol. 2020; 115(7): 1003–1006. doi:10.14309/ajg.0000000000000691.
5.
Oshima T, Siah KTH, Yoshimoto T, et al. Impacts of the COVID-19 pandemic on functional dyspepsia and irritable bowel syndrome: A population-based survey. J Gastroenterol Hepatol. 2021; 36(7): 1820–1827. doi:0.1111/jgh.15346.
6.
Vodnar DC, Mitrea L, Teleky BE, et al. Coronavirus Disease (COVID-19) Caused by (SARS-CoV-2) Infections: A Real Challenge for Human Gut Microbiota. Front Cell Infect Microbiol. 2020; 10: 575–559. doi:10.3389/fcimb.2020.575559.
7.
Segal JP, Mak JWY, Mullish BH, et al. The gut microbiome: an under-recognised contributor to the COVID-19 pandemic? Therap Adv Gastroenterol. 2020; 13: 1756284820974914. doi:10.1177/1756284820974914.
8.
Shang W, Yang Y, Rao Y, et al. The outbreak of SARS-CoV-2 pneumonia calls for viral vaccines. NPJ Vaccines. 2020; 5(1): 18. doi:10.1038/s41541-020-0170-0.
9.
Sreepadmanabh M, Sahu AK, Chande A. COVID-19: Advances in diagnostic tools, treatment strategies, and vaccine development. J Biosci. 2020; 45(1): 148. doi:10.1007/s12038-020-00114-6.
10.
Azer SA. COVID-19: pathophysiology, diagnosis, complications and investigational therapeutics. New Microbes New Infect. 2020; 37: 100738. doi:10.1016/j.nmni.2020.100738.
11.
Wirth R, Becker C, Djukic M, et al. COVID-19 im Alter – Die geriatrische Perspektive [COVID-19 in old age-The geriatric perspective]. Z Gerontol Geriatr. 2021; 54(2): 152–160. doi:10.1007/s00391-021-01864-0.
12.
Lièvre A, Turpin A, Ray-Coquard I, et al. Risk factors for Coronavirus Disease 2019 (COVID-19) severity and mortality among solid cancer patients and impact of the disease on anticancer treatment: A French nationwide cohort study (GCO-002 CACOVID-19). Eur J Cancer. 2020; 141: 62–81. doi:10.1016/j.ejca.2020.09.035.
13.
Ajaimy M, Melamed ML. COVID-19 in Patients with Kidney Disease. Clin J Am Soc Nephrol. 2020; 15(8): 1087–1089. doi:10.2215/CJN.09730620.
14.
Dinakaran D, Manjunatha N, Naveen Kumar C, et al. Neuropsychiatric aspects of COVID-19 pandemic: A selective review. Asian J Psychiatr. 2020; 53: 102188. doi:10.1016/j.ajp.2020.102188.
15.
Yelin D, Wirtheim E, Vetter P, et al. Long-term consequences of COVID-19: research needs. Lancet Infect Dis. 2020; 20(10): 1115–1117. doi:10.1016/S1473-3099(20)30701-5.
16.
Janiri D, Kotzalidis GD, Giuseppin G, et al. Gemelli Against COVID-19 Post-acute Care Study Group. Psychological Distress After Covid-19 Recovery: Reciprocal Effects With Temperament and Emotional Dysregulation. An Exploratory Study of Patients Over 60 Years of Age Assessed in a Post-acute Care Service. Front Psychiatry. 2020; 11: 590135. doi:10.3389/fpsyt.2020.59013511.
17.
Carfì A, Bernabei R, Landi F. Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA. 2020; 324(6): 603–605. doi:10.1001/jama.2020.12603.
18.
Lamers MM, Beumer J, van der Vaart J, et al. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020; 369(6499): 50–54. doi:10.1126/science.abc1669.
19.
Scialo F, Daniele A, Amato F, et al. ACE2: The Major Cell Entry Receptor for SARSCoV-2. Lung. 2020; 198(6): 867–877. doi:10.1007/s00408-020-00408-4.
20.
Garg M, Angus PW, Burrell LM. Review article: the pathophysiological roles of the renin-angiotensin system in the gastrointestinal tract. Aliment Pharmacol Ther. 2012; 35(4): 414–28. doi:10.1111/j.1365-2036.2011.04971.x.
21.
Fändriks L. The angiotensin II type 2 receptor and the gastrointestinal tract. J Renin Angiotensin Aldosterone Syst. 2010; 11(1): 43–8. doi:10.1177/1470320309347788.
22.
Hashimoto T, Perlot T, Rehman A, et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012; 487(7408): 477–81.doi:10.1038/nature11228.
23.
Wang GD, Wang XY, Hu HZ. Angiotensin receptors and actions in guinea pig enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2015; 289(3): G614–26. doi:10.1152/ajpgi.00119.2005.
24.
Pietrzak A, Skrzydło-Radomańska B, Mulak A. Rekomendacje diagnostyczno-terapeutyczne w zespole jelita nadwrażliwego. Gastroenterology Rev. 2018, 13(4): 167–196.
25.
Rządkowska D. Zespół jelita drażliwego oraz nieswoiste choroby zapalne jelit. Współ Diet. 2019; 22: 19–23.
26.
Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers. 2016; 2: 16014. doi:10.1038/nrdp.2016.14.
27.
Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014; 6: 71–80. doi:10.2147/CLEP.S40245.
28.
Oka P, Parr H, Barberio B. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020; 5(10): 908–917. doi:10.1016/S2468 1253(20)30217-X12.
29.
Nehring P, Mrozikiewicz-Rakowska B, Krasnodębski P. Irritable bowel syndrome – A new approach to a etiopathogenesis. Prz Gastroenterol. 2011; 6: 17–22.
30.
Drossman DA. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology. 2016; S0016–5085(16): 223–227. doi:10.1053/j.gastro.2016.02.032.
31.
Agirman G, Yu KB, Hsiao EY. Signaling inflammation across the gut-brain axis. Science. 2021; 374(6571): 1087–1092. doi:10.1126/science.abi6087.
32.
Simrén M, Barbara G, Flint HJ, et al. Rome Foundation Committee. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013; 62(1): 159–76. doi:10.1136/gutjnl-2012-302167
33.
Weaver KR, Melkus GD, Henderson WA. Irritable Bowel Syndrome. Am J Nurs. 2017; 117(6): 48–55. doi:10.1097/01.NAJ.0000520253.57459.01.
34.
Spiller RC. Hidden Dangers of Antibiotic Use: Increased Gut Permeability Mediated by Increased Pancreatic Proteases Reaching the Colon. Cell Mol Gastroenterol Hepatol. 2018; 6(3): 347–348. doi:10.1016/j.jcmgh.2018.06.005.
35.
Pittayanon R, Lau JT, Yuan Y, et al. Gut Microbiota in Patients With Irritable Bowel Syndrome-A Systematic Review. Gastroenterology. 2019; 157(1): 97–108. doi:10.1053/j.gastro.2019.03.049.
36.
Cuomo R, Andreozzi P, Zito FP, et al. Irritable bowel syndrome and food interaction. World J Gastroenterol. 2014; 20(27): 8837–8845. doi:10.3748/wjg.v20.i27.8837.
37.
El-Salhy M, Patcharatrakul T, Gonlachanvit S. The role of diet in the pathophysiology and management of irritable bowel syndrome. Indian J Gastroenterol. 2021; 40(2): 111–119. doi:10.1007/s12664-020-01144-6.
38.
Liu J, Chey WD, Haller E, et al. Low-FODMAP Diet for Irritable Bowel Syndrome: What We Know and What We Have Yet to Learn. Annu Rev Med. 2020; 71: 303–314. doi:10.1146/annurev-med-050218-013625.
39.
Gibson PR, Shepherd SJ. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J Gastroenterol Hepatol. 2010; 25(2): 252–258. doi:10.1111/j.1440-1746.2009.06149.x13.
40.
Pedersen N, Ankersen DV, Felding M, et al. Low-FODMAP diet reduces irritable bowel symptoms in patients with inflammatory bowel disease. World J Gastroenterol. 2017; 23(18): 3356–3366. doi:10.3748/wjg.v23.i18.3356.
41.
Nanayakkara WS, Skidmore PM, O’Brien L, et al. Efficacy of the low FODMAP diet for treating irritable bowel syndrome: the evidence to date. Clin Exp Gastroenterol. 2016; 9: 131–142. doi:10.2147/CEG.S86798.
42.
Bellini M, Tonarelli S, Nagy AG, et al. Low FODMAP Diet: Evidence, Doubts, and Hopes. Nutrients. 2020; 12(1): 148. doi:10.3390/nu12010148.
43.
Varjú P, Farkas N, Hegyi P, et al. Low fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) diet improves symptoms in adults suffering from irritable bowel syndrome (IBS) compared to standard IBS diet: A meta-analysis of clinical studies. PLoS One. 2017; 12(8): e0182942. doi:10.1371/journal.pone.0182942.
44.
van Lanen AS, de Bree A, Greyling A. Efficacy of a low-FODMAP diet in adult irritable bowel syndrome: a systematic review and meta-analysis. Eur J Nutr. 2021; 60(6): 3505–3522. doi:10.1007/s00394-020-02473-0.
45.
Staudacher HM, Whelan K. The low FODMAP diet: recent advances in understanding its mechanisms and efficacy in IBS. Gut. 2017; 66(8): 1517–1527. doi:10.1136/gutjnl-2017-313750.
46.
McIntosh K, Reed DE, Schneider T, et al. FODMAPs alter symptoms and the metabolome of patients with IBS: a randomised controlled trial. Gut. 2017; 66(7): 1241–1251. doi:10.1136/gutjnl-2015-311339.
47.
Altobelli E, Del Negro V, Angeletti PM, et al. Low-FODMAP Diet Improves Irritable Bowel Syndrome Symptoms: A Meta-Analysis. Nutrients. 2017; 9(9): 940. doi:10.3390/nu9090940.
48.
Whelan K, Martin LD, Staudacher HM, et al. The low FODMAP diet in the management of irritable bowel syndrome: an evidence-based review of FODMAP restriction, reintroduction and personalisation in clinical practice. J Hum Nutr Diet. 2018; 31(2): 239–255. doi:10.1111/jhn.12530.
49.
Guidelines FAO/WHO. Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria. Report of a Joint FAO/WHO Expert.
50.
Zawistowska-Rojek A, Tyski S. Are Probiotic Really Safe for Humans? Pol J Microbiol. 2018; 67(3): 251–258. doi:10.21307/pjm-2018-044.
51.
Sanders ME, Merenstein DJ, Reid G, et al. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat Rev Gastroenterol Hepatol. 2019; 16(10): 605–616. doi:10.1038/s41575-019-0173-3.
52.
Doron S, Snydman DR. Risk and safety of probiotics. Clin Infect Dis. 2015; 60 Suppl 2(Suppl 2): 129–134. doi:10.1093/cid/civ085.
53.
Dale HF, Rasmussen SH, Asiller ÖÖ, et al. Probiotics in Irritable Bowel Syndrome: An Up-to-Date Systematic Review. Nutrients. 2019; 11(9): 20–48. doi:10.3390/nu11092048.
54.
Sanders ME, Akkermans LM, Haller D, et al. Safety assessment of probiotics for human use. Gut Microbes. 2010; 1(3): 164–185. doi:10.4161/gmic.1.3.12127.
55.
Floch MH, Walker WA, Sanders ME, et al. Recommendations for Probiotic Use – 2015 Update: Proceedings and Consensus Opinion. J Clin Gastroenterol. 2015; 49 Suppl 1: 69–73. doi:10.1097/MCG.0000000000000420.
56.
Ducrotté P, Sawant P, Jayanthi V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J Gastroenterol. 2012; 18(30): 4012–4218. doi:10.3748/wjg.v18.i30.4012.
57.
Boonma P, Shapiro JM, Hollister EB, et al. Probiotic VSL#3 Treatment Reduces Colonic Permeability and Abdominal Pain Symptoms in Patients With Irritable Bowel Syndrome. Front Pain Res (Lausanne). 2021; 2: 691689. doi:10.3389/fpain.2021.691689.
58.
Enck P, Zimmermann K, Menke G, et al. A mixture of Escherichia coli (DSM 17252) and Enterococcus faecalis (DSM 16440) for treatment of the irritable bowel syndrome—a randomized controlled trial with primary care physicians. Neurogastroenterol Motil. 2008; 20(10): 1103–1109. doi:10.1111/j.1365-2982.2008.01156.x.
59.
Skrzydło-Radomańska B, Prozorow-Król B, Cichoż-Lach H, et al. The Effectiveness and Safety of Multi-Strain Probiotic Preparation in Patients with Diarrhea-Predominant Irritable Bowel Syndrome: A Randomized Controlled Study. Nutrients. 2021; 13(3): 756. doi:10.3390/nu1303075615.
60.
Ford AC, Harris LA, Lacy BE, et al. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018; 48(10): 1044–1060. doi:10.1111/apt.15001.
61.
Sun JR, Kong CF, Qu XK, et al. Efficacy and safety of probiotics in irritable bowel syndrome: A systematic review and meta-analysis. Saudi J Gastroenterol. 2020; 26(2): 66–77. doi:10.4103/sjg.SJG_384_19.
62.
Asha MZ, Khalil SFH. Efficacy and Safety of Probiotics, Prebiotics and Synbiotics in the Treatment of Irritable Bowel Syndrome: A systematic review and meta-analysis. Sultan Qaboos Univ Med J. 2020; 20(1): 13–24. doi:10.18295/squmj.2020.20.01.003.
63.
Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic, Biological Psychiatry. 2013; 74(10): 720–726. doi:10.1016/j.biopsych.2013.05.001.
64.
Sudo N, Chida Y, Aiba Y, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004; 558(Pt 1): 263–275. doi:10.1113/jphysiol.2004.063388.
65.
Ancona A, Petito C, Iavarone I, et al. The gut-brain axis in irritable bowel syndrome and inflammatory bowel disease. Dig Liver Dis. 2021; 53(3): 298–305. doi:10.1016/j.dld.2020.11.026.
66.
Klarin B, Johansson ML, Molin G, et al. Adhesion of the probiotic bacterium Lactobacillus plantarum 299v onto the gut mucosa in critically ill patients: a randomised open trial. Crit Care. 2005; 9(3): 285–293. doi:10.1186/cc3522.
67.
Mangell P, Nejdfors P, Wang M, et al. Lactobacillus plantarum 299v inhibits Escherichia coli-induced intestinal permeability. Dig Dis Sci. 2002; 47(3): 511–516.
68.
Pathmakanthan S, Li CK, Cowie J, et al. Lactobacillus plantarum 299: beneficial in vitro immunomodulation in cells extracted from inflamed human colon. J Gastroenterol Hepatol. 2004; 19(2): 166–173. doi:10.1111/j.1440 1746.2004.03181.x.
69.
Pinto-Sanchez MI, Hall GB, Ghajar K, et al. Probiotic bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with 16 irritable bowel syndrome. Gastroenterology 2017; 153: 448 459. doi:10.1053/j.gastro.2017.05.003.
70.
Carfì A, Bernabei R, Landi F, et al. Symptoms in Patients After Acute COVID-19. JAMA. 2020; 324(6): 603–605. doi:10.1001/jama.2020.12603.
71.
Hojyo S, Uchida M, Tanaka K, et al. How COVID-19 induces cytokine storm with high mortality. Inflamm Regen. 2020; 40: 37. doi:10.1186/s41232-020-00146-3.
72.
Nazarewska A, Lewandowski K, Kaniewska M, et al. Irritable bowel syndrome following COVID-19: underestimated consequence of infection with SARS-CoV-2. Pol Arch Intern Med. 2022: 16323. doi: 10.20452/pamw.16323.
73.
Redd WD, Zhou JC, Hathorn KE, et al. Prevalence and Characteristics of Gastrointestinal Symptoms in Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection in the United States: A Multicenter Cohort Study. Gastroenterology. 2020; 159(2): 765–767.e2. doi:10.1053/j.gastro.2020.04.045.
Udostępnij
ARTYKUŁ POWIĄZANY
Przetwarzamy dane osobowe zbierane podczas odwiedzania serwisu. Realizacja funkcji pozyskiwania informacji o użytkownikach i ich zachowaniu odbywa się poprzez dobrowolnie wprowadzone w formularzach informacje oraz zapisywanie w urządzeniach końcowych plików cookies (tzw. ciasteczka). Dane, w tym pliki cookies, wykorzystywane są w celu realizacji usług, zapewnienia wygodnego korzystania ze strony oraz w celu monitorowania ruchu zgodnie z Polityką prywatności. Dane są także zbierane i przetwarzane przez narzędzie Google Analytics (więcej).
Możesz zmienić ustawienia cookies w swojej przeglądarce. Ograniczenie stosowania plików cookies w konfiguracji przeglądarki może wpłynąć na niektóre funkcjonalności dostępne na stronie.
Możesz zmienić ustawienia cookies w swojej przeglądarce. Ograniczenie stosowania plików cookies w konfiguracji przeglądarki może wpłynąć na niektóre funkcjonalności dostępne na stronie.