REVIEW PAPER
Ochratoxin A, deoxynivalenol, T-2 and HT-2 toxins – occurrence in food and their effect on the human body
1
Katedra i Zakład Żywienia Człowieka i Metabolomiki, Pomorski Uniwersytet Medyczny w Szczecinie, Polska
Corresponding author
Karolina Patrycja Jakubczyk
Pomorski Uniwersytet Medyczny w Szczecinie, Katedra i Zakład Żywienia Człowieka i Metabolomiki,, Broniewskiego, 46, 71-460, SZCZECIN, Polska
Pomorski Uniwersytet Medyczny w Szczecinie, Katedra i Zakład Żywienia Człowieka i Metabolomiki,, Broniewskiego, 46, 71-460, SZCZECIN, Polska
Med Og Nauk Zdr. 2021;27(2):117-120
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
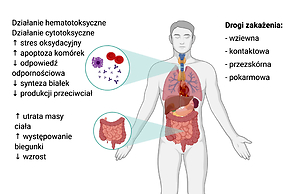
Mycotoxins are secondary metabolites of filamentous fungi. Occurring in food, they pose a threat to the health of consumers, being highly toxic substances. The effects associated with acute poisoning or chronic moderate exposure to fungal toxins are mainly based on cytotoxic, carcinogenic and inducing inflammation and oxidative stress in the organism. The aim of the review was to summarize the available information on the occurrence of ochratoxin A, deoxynivalenol and T-2 and HT-2 toxins in food and the effects on the human body.
State of knowledge:
Ochratoxin A is a common toxin recognized by the International Agency for Research on Cancer as potentially carcinogenic. The food groups most exposed to contamination are cereal products, grapes, coffee and cocoa. Deoxynivalenol, produced by soil fungi of the genus Fusarium, is mainly found in grain products – wheat, oats, barley, rye and rice. Its excessive exposure is associated with gastrointestinal symptoms, including severe vomiting and diarrhea. Exposure to mycotoxins occurring in food can be reduced by maintaining optimamum conditions for storing raw materials, sterile production line, disinfecting feed and food, and reducing bioavailability, thanks to the use of absorbents. Additionally, as demonstrated with T-2 and HT-2 toxins, the effects of mycotoxin exposure can be counteracted by increasing antioxidant intake.
Conclusion:
Food contamination with mycotoxins is a serious problem in food processing. It seems appropriate to set standards for acceptable levels of mycotoxins for a wider group of food products.
Mycotoxins are secondary metabolites of filamentous fungi. Occurring in food, they pose a threat to the health of consumers, being highly toxic substances. The effects associated with acute poisoning or chronic moderate exposure to fungal toxins are mainly based on cytotoxic, carcinogenic and inducing inflammation and oxidative stress in the organism. The aim of the review was to summarize the available information on the occurrence of ochratoxin A, deoxynivalenol and T-2 and HT-2 toxins in food and the effects on the human body.
State of knowledge:
Ochratoxin A is a common toxin recognized by the International Agency for Research on Cancer as potentially carcinogenic. The food groups most exposed to contamination are cereal products, grapes, coffee and cocoa. Deoxynivalenol, produced by soil fungi of the genus Fusarium, is mainly found in grain products – wheat, oats, barley, rye and rice. Its excessive exposure is associated with gastrointestinal symptoms, including severe vomiting and diarrhea. Exposure to mycotoxins occurring in food can be reduced by maintaining optimamum conditions for storing raw materials, sterile production line, disinfecting feed and food, and reducing bioavailability, thanks to the use of absorbents. Additionally, as demonstrated with T-2 and HT-2 toxins, the effects of mycotoxin exposure can be counteracted by increasing antioxidant intake.
Conclusion:
Food contamination with mycotoxins is a serious problem in food processing. It seems appropriate to set standards for acceptable levels of mycotoxins for a wider group of food products.
REFERENCES (33)
1.
Calvo AM, Cary JW. Association of fungal secondary metabolism and sclerotial biology. Front Microbiol. 2015; 6. doi: 10.3389/fmicb.2015.00062.
2.
Grintzalis K, Vernardis SI, Klapa MI, et al. Role of Oxidative Stress in Sclerotial Differentiation and Aflatoxin B1 Biosynthesis in Aspergillus flavus. Appl Environ Microbiol. 2014; 80: 5561–71.
3.
Mruczyk K, Jeszka J. Porównanie zawartości ochratoksyny A (OTA) i zearalenonu (ZEA) w produktach zbożowych z upraw ekologicznych i konwencjonalnych. Nauka Przyroda Technologie Uniwersytet Przyrodniczy w Poznaniu. 2013; 07.
4.
Bills GF, Gloer JB. Biologically Active Secondary Metabolites from the Fungi. Microbiol Spectr. 2016; 4. doi: 10.1128/microbiolspec.FUNK-0009-2016.
5.
Marin S, Ramos AJ, Cano-Sancho G, et al. Mycotoxins: occurrence, toxicology, and exposure assessment. Food Chem Toxicol. 2013; 60: 218–37.
6.
Chunmei J, Junling S, Qi’an H, et al. Occurrence of toxin-producing fungi in intact and rotten table and wine grapes and related influencing factors. Food Control. 2013; 31: 5–13.
7.
Gil L, Ruiz P, Font G, et al. An overview of the applications of hazards analysis and critical control point (HACCP) system to mycotoxins. Revista de Toxicología. 2016; 33: 50–5.
8.
Rai A, Das M, Tripathi A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Critical Reviews in Food Science and Nutrition. 2020; 60: 2710–29.
9.
Gruber-Dorninger C, Novak B, Nagl V, et al. Emerging Mycotoxins: Beyond Traditionally Determined Food Contaminants. J Agric Food Chem. 2017; 65: 7052–70.
10.
Degen GH. [Mycotoxins in food: Occurrence, importance and health risk. Bundesgesundheitsblatt Gesundheitsforschung Gesund-heitsschutz. 2017; 60: 745–56.
11.
Čolović R, Puvača N, Cheli F, et al. Decontamination of Mycotoxin-Contaminated Feedstuffs and Compound Feed. Toxins (Basel). 2019; 11. doi: 10.3390/toxins11110617.
12.
Adhikari M, Negi B, Kaushik N, et al. T-2 mycotoxin: toxicological effects and decontamination strategies. Oncotarget. 2017; 8: 33933–52.
13.
Liew W-P-P, Mohd-Redzwan S. Mycotoxin: Its Impact on Gut Health and Microbiota. Front Cell Infect Microbiol. 2018; 8. doi: 10.3389/fcimb.2018.00060.
14.
Klarić MŠ, Rašić D, Peraica M. Deleterious Effects of Mycotoxin Combinations Involving Ochratoxin A. Toxins (Basel). 2013; 5: 1965–87.
15.
Organization WH, Cancer IA for R on. Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Human.s 1993; 56.
16.
Malir F, Ostry V, Pfohl-Leszkowicz A, et al. Ochratoxin A: 50 Years of Research. Toxins (Basel). 2016; 8. doi: 10.3390/toxins8070191.
17.
Heussner AH, Bingle LEH. Comparative Ochratoxin Toxicity: A Review of the Available Data. Toxins (Basel). 2015; 7: 4253–82.
18.
Piotrowska M, Masek A. Saccharomyces Cerevisiae Cell Wall Components as Tools for Ochratoxin A Decontamination. Toxins. 2015; 7: 1151–62.
19.
Tao Y, Xie S, Xu F, et al. Ochratoxin A: Toxicity, oxidative stress and metabolism. Food Chem Toxicol. 2018; 112: 320–31.
20.
Zhang H, Apaliya MT, Mahunu GK, et al. Control of ochratoxin A-producing fungi in grape berry by microbial antagonists: A review. Trends in Food Science & Technology. 2016; 51: 88–97.
21.
Wang Y, Wang L, Liu F, et al. Ochratoxin A Producing Fungi, Biosynthetic Pathway and Regulatory Mechanisms. Toxins (Basel). 2016; 8. doi: 10.3390/toxins8030083.
22.
Kőszegi T, Poór M. Ochratoxin A: Molecular Interactions, Mechanisms of Toxicity and Prevention at the Molecular Level. Toxins (Basel). 2016; 8. doi: 10.3390/toxins8040111.
23.
Poór M, Veres B, Jakus PB, et al. Flavonoid diosmetin increases ATP levels in kidney cells and relieves ATP depleting effect of ochratoxin A. J Photochem Photobiol B, Biol. 2014; 132: 1–9.
24.
Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs (Text with EEA relevance). OJ L. 2006; 364.
25.
Statement on recent scientific information on the toxicity of Ochratoxin A. EFSA Journal. 2010; 8: 1626.
26.
Statement on the risks for public health related to a possible increase of the maximum level of deoxynivalenol for certain semi-processed cereal products. EFSA Journal. 2013; 11: 3490.
27.
Knutsen HK, Alexander J, Barregård L, et al. Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA Journal. 2017; 15: e04718.
28.
Liang H, Wang X, Yan J, et al. Characterizing the Intra-Vineyard Variation of Soil Bacterial and Fungal Communities. Front Microbiol. 2019; 10. doi: 10.3389/fmicb.2019.01239.
29.
Scientific Opinion on the risks for animal and public health related to the presence of T-2 and HT-2 toxin in food and feed. EFSA Journal. 2011; 9: 2481.
30.
Yang L, Tu D, Wang N, et al. The protective effects of DL-Selenomethionine against T-2/HT-2 toxins-induced cytotoxicity and oxidative stress in broiler hepatocytes. Toxicol In Vitro. 2019; 54: 137–46.
31.
Nathanail AV, Varga E, Meng-Reiterer J, et al. Metabolism of the Fusarium Mycotoxins T-2 Toxin and HT-2 Toxin in Wheat. J Agric Food Chem. 2015; 63: 7862–72.
32.
Human and animal dietary exposure to T-2 and HT-2 toxin. European Food Safety Authority. 2017.
33.
Dai C, Xiao X, Sun F, et al. T-2 toxin neurotoxicity: role of oxidative stress and mitochondrial dysfunction. Arch Toxicol. 2019; 93: 3041–56.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.