RESEARCH PAPER
Post-vaccination reactions occurring in patients vaccinated against SARS-COV-2
1
Zakład Gerontologii, Katedra Zdrowia Publicznego, Wydział Nauk o Zdrowiu, Uniwersytet Medyczny we Wrocławiu, Polska
2
Wyższa Szkoła Medyczna w Kłodzku, Polska
3
Państwowa Wyższa Szkoła Techniczno- Ekonomiczna im. ks. B. Markiewicza w Jarosławiu, Polska
4
Zakład Fizjopatologii, Instytut Medycyny Wsi w Lublinie, Polska
5
Zakład Antropologii Medycznej, Instytut Medycyny Wsi w Lublinie, Polska
6
Wyższa Szkoła Rehabilitacji w Warszawie, Polska
Corresponding author
Piotr Choina
Zakład Antropologii Medycznej, Instytut Medycyny Wsi w Lublinie, ul. Jaczewskiego 2, 20-090, Lublin, Polska
Zakład Antropologii Medycznej, Instytut Medycyny Wsi w Lublinie, ul. Jaczewskiego 2, 20-090, Lublin, Polska
Med Og Nauk Zdr. 2021;27(4):421-427
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
Vaccinations are the safest and for many years proven way of acquiring immunity. In the European Union, two mRNA vaccines and two vector vaccines based on modified adenoviruses have been registered for the prevention of COVID-19. Adverse vaccine reactions (AVRs) are any abnormal body reaction or event after vaccination. These reactions can range in severity from mild to rarely occurring serious or even life-threatening. The aim of the study was to evaluate adverse vaccine reactions (AVRs) in patients vaccinated against SARS-COV-2.
Material and methods:
The study was conducted among 376 people using the diagnostic survey method. The inclusion criterion was vaccination against SARS-CoV-2 virus. Due to the lack of standardized tools adapted to the planned research, an author-constructed questionnaire composed of 15 items was used.
Results:
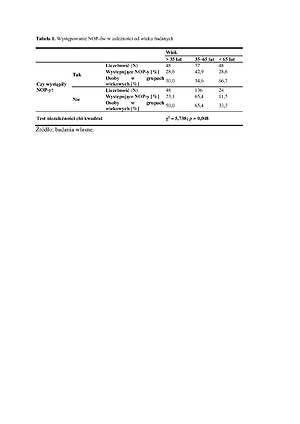
Among the respondents, 37.3% received two doses of the vaccine. No vaccine reactions occurred in 47.9% of patients. There was a significant relationship between the age of the respondents [χ2 (2; N=376)=5.74; p<0.05; V=0.24], kidney diseases (p =0.019), the presence of arterial hypertension (p=0.0003), neoplastic diseases (p=0.047), chronic respiratory diseases (p =0.015), the amount of medications taken [χ2 (2; N=376)=5.62; p<0.05; V=0.23], with successive doses of two- dose vaccination [χ2 (2; N=376)=4.23; p<0.05; V=0.34].
Conclusions:
The anti-Covid-19 vaccines used, regardless of the mechanism of action, turned out to be safe. AVRs are more common in the elderly suffering from comorbidities and taking 3–5 medications on a regular basis. AVRs are more common after the second dose of the vaccine
Vaccinations are the safest and for many years proven way of acquiring immunity. In the European Union, two mRNA vaccines and two vector vaccines based on modified adenoviruses have been registered for the prevention of COVID-19. Adverse vaccine reactions (AVRs) are any abnormal body reaction or event after vaccination. These reactions can range in severity from mild to rarely occurring serious or even life-threatening. The aim of the study was to evaluate adverse vaccine reactions (AVRs) in patients vaccinated against SARS-COV-2.
Material and methods:
The study was conducted among 376 people using the diagnostic survey method. The inclusion criterion was vaccination against SARS-CoV-2 virus. Due to the lack of standardized tools adapted to the planned research, an author-constructed questionnaire composed of 15 items was used.
Results:
Among the respondents, 37.3% received two doses of the vaccine. No vaccine reactions occurred in 47.9% of patients. There was a significant relationship between the age of the respondents [χ2 (2; N=376)=5.74; p<0.05; V=0.24], kidney diseases (p =0.019), the presence of arterial hypertension (p=0.0003), neoplastic diseases (p=0.047), chronic respiratory diseases (p =0.015), the amount of medications taken [χ2 (2; N=376)=5.62; p<0.05; V=0.23], with successive doses of two- dose vaccination [χ2 (2; N=376)=4.23; p<0.05; V=0.34].
Conclusions:
The anti-Covid-19 vaccines used, regardless of the mechanism of action, turned out to be safe. AVRs are more common in the elderly suffering from comorbidities and taking 3–5 medications on a regular basis. AVRs are more common after the second dose of the vaccine
REFERENCES (34)
1.
Jackson NAC, Kester KE, Casimiro D, Gurunathan S, DeRosa F. The Promise of mRNA Vaccines: A Biotech and Industrial Perspective. Npj Vaccines. 2020; 5(1): 1–6.
2.
Maruggi G, Zhang C, Li J, Ulmer JB, Yu D.: mRNA as a Transformative Technology for Vaccine Development to Control Infectious Diseases. Molecular Therapy. 2019; 27(4): 757–72.
3.
Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA Vaccines — a New Era in Vaccinology. Nature Reviews. Drug Discovery. 2018;17(4): 261–279.
4.
Jackson LA, Anderson EJ, Rouphae NG, Roberts PC, Makhene M. et al. An mRNA Vaccine against SARS-CoV-2 – Preliminary Report. N Engl J Med. 2020; 383(20): 1920–1931.
5.
Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A. et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020; 586(7830): 589–593.
6.
Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM. et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2020; S0140–6736(20):32661–1.
7.
Hu X, Shrimp JH, Guo H, Xu M, Chen CZ, Zhu W, et al. Discovery of TMPRSS2 Inhibitors from Virtual Screening as a Potential Treatment of COVID-19. ACS Pharmacology and Translational Science. https:// doi.org/10.1021/acsptsci.0c00221, access: 21–06–2021.
8.
Chan JF-W, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020; S0140–6736(20): 30154–9.
9.
Szczuka I. Niepożądane odczyny po szczepieniu BCG w Polsce w latach 1994–2000. Przegląd Epidemiologiczny 2002; 56: 205–216.
10.
Koperny M, Bała M, Bandoła K, Seweryn M, Żak J. Analiza występowania niepożądanych odczynów poszczepiennych w Polsce w latach 2003–2012. Probl Hig Epidemiol. 2014; 95(3): 609.
11.
Błędowski P, Szatur-Jaworska B, Szweda-Lewandowska Z, Kubick P. Raport na temat sytuacji osób starszych w Polsce. Warszawa: Instytut Pracy i Spraw Socjalnych; 2012. p. 107.
12.
Gates BJ, Walker KM. Fizjologiczne zmiany u osób starszych i ich wpływ na leczenie cukrzycy. Diabetologia po Dyplomie 2014; 11(1): 17–27.
13.
Majsnerowska A, Poloński L. Ostry zespół wieńcowy u osób w podeszłym wieku. Choroby Serca i Naczyń. 2017; 14(1): 15–23.
14.
Gnjidic D, Hilmer SN, Blyth FM, et al. Polypharmacy cutoff and outco-mes: five or more medicines were used to identify communitydwelling older men at risk of different adverse outcomes. J Clin Epidemiol. 2012; 65(9): 989–95.
15.
Mastromarino V, Casenghi M, Testa M, et al. Polypharmacy in heart failure patients. Curr Heart Fail Rep. 2014; 11(2): 212–9.
16.
Wardzyńska A, Kowalski M. Starzenie się układu odpornościowego a alergia u osób w podeszłym wieku. Alergia Astma Immunologia 2009; 14(4): 239–247.
17.
Iwasaki A. What reinfections mean for COVID-19. Lancet Infect Dis. 2021; 21(1): 3–5.
18.
Tavares DMDS, Oliveira NGN, Marchiori GF, Guimarães MSF, San tana LPM. Elderly individuals living by themselves: knowledge and measures to prevent the novel coronavirus. Rev Lat Am Enfermagem. 2020; 6(28): e3383.
19.
Negrini ELD, Nascimento CF, Silva A, et al. Elderly persons who live alone in Brazil and their lifestyle. Rev Bras Geriatr Gerontol. 2018;21(5): 523–31.
20.
Wang C, Pan R, Wan X, et al. Immediate Psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) Epidemic among the general population in China. Int J Environ Res Public Health. 2020; 17(5): 1–25.
21.
Li R, Pei S, Chen B, Song Y, Zhang T, Yang W. Shaman J. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science 2020; 368(6490): 489–493.
22.
Cunningham-Rundles C: Physiology of IgA and IgA deficiency. J Clin Immunol. 2001; 21(5): 303–309.
23.
Lu R, Zhao X, Li J, Peihua Niu P, Yang B, Wu H, et al. Genomic charac terisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020; 395(10224): 565–574.
24.
Komunikat Ministersta Zdrowia nr 14 w sprawieszczepień przeciw COVID-10 dawką przypominającą oraz dawką dodatkową uzupełniającą schemat podstawowy. Warszawa, 27.10.2021.
25.
Wolf MS, Serper M, Opsasnick L, et al. Awareness, Attitudes, and Actions Related to COVID-19 Among Adults With Chronic Conditions at the Onset of the U.S. Outbreak: A Cross-sectional Survey. Ann Intern Med. 2020; 173: 100–109.
26.
World Health Organisation. Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases: interim guidance, world health organisation 2020.
27.
Qazi A, Qazi J, Naseer K, et al. Analyzing situational awareness through public opinion to predict adoption of social distancing amid pandemic COVID-19. J Med Virol. 2020; 92(7): 849–855.
28.
Winichakoon P, Chaiwarith R, Liwsrisakun C, et al. Negative nasop - haryngeal and oropharyngeal swabs do not rule out COVID-19. J Clin Microbiol. 2020; 58: 1–3.
29.
Wölfel R, Corman V, Guggemos W, Seilmaier M, Zange S, Müller M, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020; 581: 465–469.
30.
Zawilska J, Kuczyńska K, Gawior M, Kosiorek M, Dąbrowska K, Dominiak Z, et al. Szczepionki i leki stosowane w terapii COVID-19. Farmacja Polska. 2021; 77(2): 178–192.
31.
Ogolodom MP, Mbaba AN, Alazigha N, et al. Knowledge, Attitudes and Fears of HealthCare Workers towards the Corona Virus Disease (COVID-19) Pandemic in South-South, Nigeria. Health Sci J. 2020; 1: 002.
32.
McCormack LA, Squiers L, Frasier AM, et al. Gaps in Knowledge About COVID-19 Among US Residents Early in the Outbreak. Public Health Rep. 2021; 136(1): 107–116.
33.
Barber SJ, Kim H. COVID-19 Worries and Behavior Changes in Older and Younger Men and Women. J Gerontol B Psychol Sci Soc Sci. 2020;1–7.
34.
Matuschek C, Moll F, Fangerau H, et al. Face masks: benefits and risks during the COVID-19 crisis. Eur J Med Res. 2020; 25: 32.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.