REVIEW PAPER
Influenza: state of knowledge, treatment and prevention
1
Katedra i Zakład Mikrobiologii Lekarskiej, Warszawski Uniwersytet Medyczny, Polska
Corresponding author
Anna Majewska
Katedra i Zakład Mikrobiologii Lekarskiej, Warszawski Uniwersytet Medyczny, ul. Chałubińskiego 5, 02-004, Warszawa, Polska
Katedra i Zakład Mikrobiologii Lekarskiej, Warszawski Uniwersytet Medyczny, ul. Chałubińskiego 5, 02-004, Warszawa, Polska
Med Og Nauk Zdr. 2021;27(3):220-226
KEYWORDS
TOPICS
Biomedyczne aspekty zdrowia i chorobyZdrowie publiczneProfilaktyka chorób i promocja zdrowiaEpidemiologia
ABSTRACT
Introduction and objective:
For centuries influenza has been one of the most prevalent viral infectious diseases and still remains an important cause of morbidity and mortality in many regions of the world, including Poland. The aim of the study was presentation of the scope of problems concerning influenza virus infections and the available options for controlling the disease.
Review methods:
The publication was prepared based on a literature review in scientific information databases and on the websites of organizations operating in the field of public health. As part of the issue analysis, a systematic search was performed of current scientific data concerning the described problem.
Brief description of the state of knowledge:
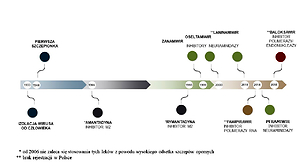
Four types of influenza virus are distinguished: A, B, C and D. Infections in humans are most often caused by influenza A and B. The best recognized virus is influenza A, which is characterized by high antigenic and genetic variability and zoonotic potential. Influenza A virus is responsible for local epidemics and pandemics and meets all the criteria of a pathogen that can cause a catastrophic biological threat on a global scale. Modern medicine has the ability to control influenza through protective vaccinations with multivalent vaccines and antiviral drugs, which include neuraminidase inhibitors and viral polymerase inhibitors.
Summary:
Influenza is a major public health risk problem. The actual risk of a global epidemic in the future forces to undertake transdisciplinary and integrated actions striving at an effective immunoprophylaxis of influenza virus infections, as well as treatment of the disease and its complications. A global epidemic may go beyond the capabilities of individual countries. The development of a vaccine against pandemic influenza and its mass production in a short time has the utmost priority.
For centuries influenza has been one of the most prevalent viral infectious diseases and still remains an important cause of morbidity and mortality in many regions of the world, including Poland. The aim of the study was presentation of the scope of problems concerning influenza virus infections and the available options for controlling the disease.
Review methods:
The publication was prepared based on a literature review in scientific information databases and on the websites of organizations operating in the field of public health. As part of the issue analysis, a systematic search was performed of current scientific data concerning the described problem.
Brief description of the state of knowledge:
Four types of influenza virus are distinguished: A, B, C and D. Infections in humans are most often caused by influenza A and B. The best recognized virus is influenza A, which is characterized by high antigenic and genetic variability and zoonotic potential. Influenza A virus is responsible for local epidemics and pandemics and meets all the criteria of a pathogen that can cause a catastrophic biological threat on a global scale. Modern medicine has the ability to control influenza through protective vaccinations with multivalent vaccines and antiviral drugs, which include neuraminidase inhibitors and viral polymerase inhibitors.
Summary:
Influenza is a major public health risk problem. The actual risk of a global epidemic in the future forces to undertake transdisciplinary and integrated actions striving at an effective immunoprophylaxis of influenza virus infections, as well as treatment of the disease and its complications. A global epidemic may go beyond the capabilities of individual countries. The development of a vaccine against pandemic influenza and its mass production in a short time has the utmost priority.
REFERENCES (44)
1.
Globa l Influenza Strategy 2019–2013. Prevent, Control, Prepare. World Health Organization 2019. https://www.who.int/influenza/... -fluenza_strategy_2019_2030/en/ (dostęp 12.01.2021).
2.
Vaccines against influenza WHO position paper—November 2012. Wkly. Epidemiol. Rec. 2012; 87(47): 461–476.
3.
Rothberg M, Haessler SD. Complications of seasonal and pandemic in-fluenza. Critical Care Medicine. 2010; 38: 91–97. https://doi.org/10.1097/CCM.0b....
5.
Brydak L. Grypa – problem stary jak świat. Hygeia Public Health. 2012; 47(1): 1–7.
6.
Emerging respiratory disease – influenza virus overview. Dis Mon. 2017; 63(9): 248–251. https://doi.org/10.1016/j.disa....
7.
Lambert LC, Fauci A. Influenza vaccines for the future. N Engl J Med. 2010; 18(363): 2036–2044. https://doi.org/10.1056/NEJMra....
8.
Asha K, Kumar B. Emerging influenza D virus threat: what we know so far! J Clin Med. 2019; 8: 192. https://doi.org/10.3390/jcm802....
9.
Brydak L. Grypa znana od stuleci – nadal groźna. Fam Med Primary Care Rev. 2014; 16(2): 181–184.
10.
Hussain M, Galvin HD, Haw TY, et al. Drug resistance in influenza A virus: the epidemiology and management. Infect Drug Resist. 2017; 10:121–134.
11.
McAuley JL, Gilbertson BP, Trifkovic S, et al. Influenza virus neura -minidase structure and functions. Front Microbiol. 2019;10:39. https://doi.org/10.3389/fmicb.....
12.
Markowska-Daniel I, Mickiewicz M, Witkowski L, i wsp. Charakte-rystyka nowego wirusa grypy typu D. Med. Weter. 2016; 72: 531–535.
14.
Beigel J, Bray M. Current and future antiviral therapy of severe seaso-nal and avian influenza. Antiviral Res. 2008; 78: 91–102. https://doi.org/10.1016/j.anti....
15.
te Velthuis A, Fodor E. Influenza virus RNA polymerase: insights into the mechanisms of viral RNA synthesis. Nat Rev Microbiol. 2016; 14(8): 79–493. https://doi.org/10.1038/nrmicr....
16.
Shao W, Li Xm Goraya MU, et al. Evolution of influenza A virus by mutation and re-assortment. Int J Mol Sci. 2017; 18(8): 1650. https://doi.org/10.3390/ijms18....
17.
Bouvier N. The Future of Influenza Vaccines: A Historical and Clini -cal Perspective. Vaccines (Basel). 2018; 6: 58. https://doi.org/10.3390/vaccin....
18.
Wong JY, Kelly H, Ip DK, et al. Case fatality risk of influenza A (H1N1pdm09): a systematic review. Epidemiology. 2013; 24(6): 830–841. https://doi.org/10.1097/EDE.0b....
19.
Gaydos JC, Top F. Hodder RA, et al. Swine Influenza A outbreak, Fort Dix, New Jersey, 1976. Emerg Infect Dis. 2006; 12: 23–28. https://doi.org/10.3201/eid120....
20.
Iskander J, Strikas RA, Gensheimer KF, et al. Pandemic influenza planning, United States, 1978–2008. Emerg Infect Dis. 2013; 19(6): 879–885. https://doi.org/10.3201/eid190....
21.
Rozo M, Gronvall G. The reemergent 1977 H1N1 strain and the gain-of-Function Debate. mBio. 2015; 6(4): 1013–1015. https://doi.org/10.1128/mBio.0....
22.
Adalja AA, Watson M, Toner ES, et al. Characteristics of microbes most likely to cause pandemics and global catastrophes. In: Inglesby T., Adalja A, eds. Global Catastrophic Biological Risks. Curr. Top. Microbiol. Immunol. 2019; 424: p. 1–20. https://doi.org/10.1007/82_201....
23.
Król E, Rychłowska M, Szewczyk B. Antivirals – current trends in fighting influenza. Acta Biochim Pol. 2014; 61: 495–504.
24.
Mifsud EJ, Hayden FG, Hurt AC. Antivirals targeting the polymerase complex of influenza viruses. Antiviral Res. 2019; 169: 104545. https://doi.org/10.1016/j.anti....
25.
Scott LJ. Peramivir: A Review in Uncomplicated Influenza. Drugs. 2018; 78: 1363–1370.
26.
Influenza Antiviral Medications: Summary for Clinicians, https://www.cdc.gov/flu/profes... (dostęp 16.05.2021).
27.
Uyeki TM, Bernstein HH, Bradley JS, i wsp. Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza, Clin Infect Dis. 2019; 68, 6: 1–47. https://doi.org/10.1093/cid/ci....
28.
Zapobieganie, rozpoznawanie i leczenie grypy. Wytyczne Kolegium lekarzy Rodzinnych w Polsce, 2019 zalecane przez Konsultanta Krajowego w dziedzinie medycyny rodzinnej oraz Konsultanta Krajowego w dziedzinie chorób zakaźnych. https://www.klrwp.pl/strona/61.... (dostęp 16.05.2021).
29.
Shie JJ, Fang JM. Development of effective anti-influenza drugs: congeners and conjugates – a review. J Biomed Sci. 2019; 26(84). https://doi.org/10.1186/s12929....
30.
Kashiwagi S, Watanabe A, Ikematsu H, et al. Long-acting neuraminidase inhibitor laninamivir octanoate as post-exposure prophylaxis for influenza. Clin Infect Dis. 2016; 63(3): 330–337. https://doi.org/10.1093/cid/ci....
31.
Heo YA. Baloxavir: first global approval. Drugs. 2018; 78(6): 693–697. https://doi.org/10.1007/s40265....
32.
Ng KE. Xofluza (baloxavir marboxil) for the treatment of acute uncomplicated influenza. P T. 2019; 44(1): 9–11.
33.
Mazuchowski M, Puchała L. Baloxavir marboxil jako opcja terapeutyczna w leczeniu grypy w Polsce. Farm Pol. 2020; 76(8): 438–441.
34.
Joshi S, Parkar J, Ansari A, et al. Role of favipiravir in the treatment of COVID-19.
36.
Coomes EA, Haghbayan H. Favipiravir, an antiviral for COVID-19? J Antimicrob Chemother. 2020; 75(7): 2013–2014. https://doi.org/10.1093/jac/dk....
37.
Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. 2017; 93(7): 449–463. https://doi.org/10.2183/pjab.9....
38.
European Centre for Disease Prevention and Control. Types of seasonal influenza vaccine https://www.ecdc.europa.eu/en/... (dostęp 12.02.2021).
39.
Influenza Vaccine for 2020–2021. JAMA. 2020; 324(17): 1777–1778. https://doi.org/10.1001/jama.2....
40.
Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases (NCIRD). How influenza (flu) vaccines are made https://www.cdc.gov/flu/preven... (dostęp 12.01.2021).
41.
Dunning J, Thwaites RS, Openshaw PJM. Seasonal and pandemic influenza: 100 years of progress, still much to learn. Mucosal Immunol. 2020; 13: 566–573. https://doi.org/10.1038/s41385....
42.
Sternal D, Owsianko A. Opinie personelu medycznego na temat zalecanych szczepień przeciwko grypie. Med Og Nauk Zdr. 2019; 25(1): 16–21.
43.
Michalik A, Gawlik K. Attitudes and knowledge of Health care workers in Cieszyn County of the Silesian Province in southern Poland about seasonal flu vaccinations – preliminary study. Med Og Nauk Zdr. 2020; 1: 35–41. https://doi.org/10.26444/monz/....
44.
Seasonal influenza vaccination and antiviral use in EU/EEA Member States www.ecdc.europa.eu/sites/default/files/documents/seasonal-influenza-antiviral-use-2018.pdf. (dostęp 12.01.2021).
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.