Online first
Bieżący numer
Archiwum
O czasopiśmie
Polityka etyki publikacyjnej
System antyplagiatowy
Instrukcje dla Autorów
Instrukcje dla Recenzentów
Rada Redakcyjna
Komitet Redakcyjny
Recenzenci
Wszyscy recenzenci
2024
2023
2022
2021
2020
2019
2018
2017
2016
Kontakt
Bazy indeksacyjne
Klauzula przetwarzania danych osobowych (RODO)
PRACA PRZEGLĄDOWA
Imbir lekarski (Zingiber officinale) – surowiec o właściwościach terapeutycznych
1
Chair of Human Nutrition and Metabolomics, Pomeranian Medical University in Szczecin, Szczecin, Poland
2
Chair of Medical Chemistry, Pomeranian Medical University in Szczecin, Szczecin, Poland
Autor do korespondencji
Katarzyna Janda
Chair and Department of Human Nurtition and Metabolomics, Pomeranian Medical University, Broniewskiego 24, 71-460 Szczecin, Poland
Chair and Department of Human Nurtition and Metabolomics, Pomeranian Medical University, Broniewskiego 24, 71-460 Szczecin, Poland
Med Og Nauk Zdr. 2021;27(1):40-44
SŁOWA KLUCZOWE
imbirwłaściwości prozdrowotnewłaściwości przeciwwymiotnewłaściwości hipoglikemicznewłaściwości hipolipemizującewłaściwości przeciwzapalne
DZIEDZINY
STRESZCZENIE
Wprowadzenie i cel pracy:
Imbir (Zingiber officinale) jest rośliną szeroko stosowaną na całym świecie. Ze względu na bogaty aromat i charakterystyczny ostry smak znalazł zastosowanie w kuchni jako przyprawa. Jednak mnogość zawartych w nim fitoskładników sprawia, że imbir wykazuje również pozytywny wpływ na organizm człowieka. Celem pracy jest przedstawienie możliwości wykorzystania imbiru i jego bioaktywnych składników w leczeniu wybranych chorób.
Skrócony opis stanu wiedzy:
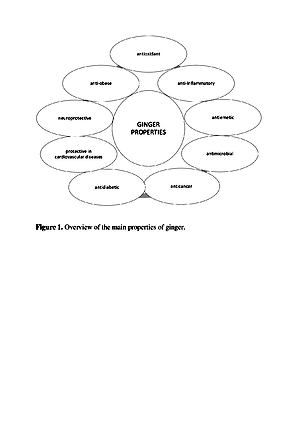
Imbir jest źródłem wielu cennych składników odżywczych, które nadają mu właściwości organoleptyczne, ale także prozdrowotne. Olejki eteryczne i oleożywica są głównymi składnikami odpowiedzialnymi za specyficzny zapach i ostry smak imbiru. Ponadto imbir zawiera wiele fitoskładników, takich jak seskwiterpeny i monoterpeny, do których należą: α-zingiberen, α-faranezen, β-bisabolen, β-felandren, zingiberol, geraniol, linalool, cynoele, a także zingeron i szogaole. Imbir jest od dawna stosowany w leczeniu zaburzeń żołądkowo-jelitowych i sercowo-naczyniowych, bólów reumatycznych, cukrzycy, nowotworów i depresji. Nadal znajduje zastosowanie w chińskiej i arabskiej medycynie ludowej jako środek rozgrzewający lub jako lek na choroby układu pokarmowego i wątroby. Ponadto stosuje się go w zaparciach, przeziębieniach, nieżytach nosa i zapaleniu oskrzeli. Badania wskazują również na cenne właściwości antyoksydacyjne, przeciwbakteryjne i przeciwzapalne imbiru. Te korzyści zdrowotne przypisuje się zawartym w nim związkom fenolowym, głównie gingerolowi i shoagolowi.
Podsumowanie:
Imbir jest bogatym źródłem wielu związków bioaktywnych, które posiadają właściwości lecznicze i mogą być stosowane wspomagająco w wielu chorobach, takich jak cukrzyca, choroby układu krążenia, nudności, wymioty i procesy zapalne.
Imbir (Zingiber officinale) jest rośliną szeroko stosowaną na całym świecie. Ze względu na bogaty aromat i charakterystyczny ostry smak znalazł zastosowanie w kuchni jako przyprawa. Jednak mnogość zawartych w nim fitoskładników sprawia, że imbir wykazuje również pozytywny wpływ na organizm człowieka. Celem pracy jest przedstawienie możliwości wykorzystania imbiru i jego bioaktywnych składników w leczeniu wybranych chorób.
Skrócony opis stanu wiedzy:
Imbir jest źródłem wielu cennych składników odżywczych, które nadają mu właściwości organoleptyczne, ale także prozdrowotne. Olejki eteryczne i oleożywica są głównymi składnikami odpowiedzialnymi za specyficzny zapach i ostry smak imbiru. Ponadto imbir zawiera wiele fitoskładników, takich jak seskwiterpeny i monoterpeny, do których należą: α-zingiberen, α-faranezen, β-bisabolen, β-felandren, zingiberol, geraniol, linalool, cynoele, a także zingeron i szogaole. Imbir jest od dawna stosowany w leczeniu zaburzeń żołądkowo-jelitowych i sercowo-naczyniowych, bólów reumatycznych, cukrzycy, nowotworów i depresji. Nadal znajduje zastosowanie w chińskiej i arabskiej medycynie ludowej jako środek rozgrzewający lub jako lek na choroby układu pokarmowego i wątroby. Ponadto stosuje się go w zaparciach, przeziębieniach, nieżytach nosa i zapaleniu oskrzeli. Badania wskazują również na cenne właściwości antyoksydacyjne, przeciwbakteryjne i przeciwzapalne imbiru. Te korzyści zdrowotne przypisuje się zawartym w nim związkom fenolowym, głównie gingerolowi i shoagolowi.
Podsumowanie:
Imbir jest bogatym źródłem wielu związków bioaktywnych, które posiadają właściwości lecznicze i mogą być stosowane wspomagająco w wielu chorobach, takich jak cukrzyca, choroby układu krążenia, nudności, wymioty i procesy zapalne.
Introduction:
Ginger (Zingiber officinale) is a plant widely used all over the world. Due to its rich aroma and characteristic, spicy taste, it has been used in the kitchen as a spice additive. However, the multitude of phytonutrients it contains makes ginger a plant with a positive effect on the human body.
Objective:
The aim of the study is to present the possibilities of using ginger and its bioactive ingredients in the treatment of selected diseases.
Brief description of the state of knowledge:
Ginger is a source of many valuable nutrients that determine its organoleptic characteristics, which also has pro-health properties. Essential oils and oleoresin are the main compounds responsible for the specific smell and sharp taste of ginger [8]. Additionally, ginger contains many phytonutrients, such as sesquiterpenes and monoterpenes, which include α – zingiberene, α – faranezene, β – bisabolene, β – felandren, zingiberol, geraniol, linalool, and cineole, as well as zingerone and shogaole. Ginger has been used for a long time to treat gastrointestinal and cardiovascular disorders, rheumatic pains, diabetes, cancer and depression. It is still used in Chinese and Arab folk medicine as a warming agent, or as a remedy for the digestive system and liver diseases. Moreover, it is used in constipation, cold, rhinitis and bronchitis. Research also indicates high antioxidant, antimicrobial and anti-inflammatory properties. These health benefits are attributed to its phenolic compounds, mainly gingerols and shoagols.
Conclusions:
Ginger is a rich source of multiple bioactive compounds which have medicinal value, and has a supporting effect in several diseases, such as diabetes, cardiovascular diseases, nausea, emesis and inflammatory processes.
Ginger (Zingiber officinale) is a plant widely used all over the world. Due to its rich aroma and characteristic, spicy taste, it has been used in the kitchen as a spice additive. However, the multitude of phytonutrients it contains makes ginger a plant with a positive effect on the human body.
Objective:
The aim of the study is to present the possibilities of using ginger and its bioactive ingredients in the treatment of selected diseases.
Brief description of the state of knowledge:
Ginger is a source of many valuable nutrients that determine its organoleptic characteristics, which also has pro-health properties. Essential oils and oleoresin are the main compounds responsible for the specific smell and sharp taste of ginger [8]. Additionally, ginger contains many phytonutrients, such as sesquiterpenes and monoterpenes, which include α – zingiberene, α – faranezene, β – bisabolene, β – felandren, zingiberol, geraniol, linalool, and cineole, as well as zingerone and shogaole. Ginger has been used for a long time to treat gastrointestinal and cardiovascular disorders, rheumatic pains, diabetes, cancer and depression. It is still used in Chinese and Arab folk medicine as a warming agent, or as a remedy for the digestive system and liver diseases. Moreover, it is used in constipation, cold, rhinitis and bronchitis. Research also indicates high antioxidant, antimicrobial and anti-inflammatory properties. These health benefits are attributed to its phenolic compounds, mainly gingerols and shoagols.
Conclusions:
Ginger is a rich source of multiple bioactive compounds which have medicinal value, and has a supporting effect in several diseases, such as diabetes, cardiovascular diseases, nausea, emesis and inflammatory processes.
Antoniewicz J, Jakubczyk K, Gutowska I, Janda K. Ginger (Zingiber officinale) – spice with therapeutic properties. Med Og Nauk Zdr. 2021; 27(1): 40–44. doi: 10.26444/monz/134013
REFERENCJE (61)
1.
Mahomoodally MF, Aumeeruddy MZ, Rengasamy K, et al. Ginger and its active compounds in cancer therapy: From folk uses to nano- therapeutic applications. Semin. Cancer Biol. 2019; S1044-579X(19): 30213–30215. doi: 10.1016/j.semcancer.2019.08.009.
2.
Semwal RB, Semwal DK, Combrinck S, et al. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry. 2015; 117: 554–568. doi: 10.1016/j.phy tochem.2015.07.012.
3.
Bodagh MN, Maleki I, Hekmatdoost A. Ginger in gastrointestinal disorders: A systematic review of Clinical trials. Food Sci Nutr. 2019; 7(1): 96 –108. doi:10.1002/fsn3/807.
4.
Braun L, Cohen M. Herbs and Natural Supplements. An Evidence- based Guide. 2nd ed. Australia: Elsevier, 2007.
6.
Banihani SA. Ginger and testosterone. Biomolecules. 2018; 8(4): 119. https://doi.org/10.3390/biom80....
7.
De Lima RMT, dos Reis AC, de Menezes A, et al. Protective and therapeutic potential of ginger Zingiber officinale extract and [6]-gingerol in cancer: A comprehensive review. Phyther Res. 2018; 32(2): 1885–1907. doi: 10.1002/ptr.6134.
8.
Huang B, Wang G, Chu Z, et al. Effect of Oven Drying, Microwave Drying, and Silica Gel Drying Methods on the Volatile Components of Ginger (Zingiber officinale Roscoe) by HS-SPME-GC-MS. Dry Technol. 2012; 30(2): 248–255. doi: 10.1080/07373937.2011.634976.
9.
Kulczyński B, Gramza-Michałowska A. Znaczenie żywieniowe imbiru. Bromatol. Chem Toksykol. 2016; 49(1): 57–63.
10.
De Lima RMT, Dos Reis AC, De Oliviera Santos JV, et al. Toxic, cytogenetics and antitumor evaluations of [6]-gingerol in non-clinical in vitro studies. Biomed. Pharmacother. 2019; 115: 108873. doi:10.1016/j.biopha.2019.108873.
11.
Zhang F. Zhang JG, Yang W, et al. 6-Gingerol attenuates LPS-induced neuroinflammation and cognitive impairment partially via suppressing astrocyte overactivation. Biomed Pharmacother. 2018; 107: 1523–1529. doi: 10.1016/j.biopha.2018.08.136.
12.
Dugasani S, Pichika MR, Nadarajah VD, et al. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J Ethnopharmacol. 2010; 127: 515–520. doi:10.1016/j.jep.2009.10.004.
13.
Abusarah J, Benabdoune H, Shi Q, et al. Elucidating the Role of Protandim and 6-Gingerol in Protection Against Osteoarthritis. J Cell Biochem. 2017; 118: 1003–1013. doi:10.1002/jcb.25659.
14.
Wang J, Zhang L, Dong L, et al. 6-Gingerol, a Functional Polyphenol of Ginger, Promotes Browning through an AMPK-Dependent Pathway in 3T3-L1 Adipocytes. J. Agric. Food Chem. 2019; 67: 14056–14065. doi: 10.1021/acs.jafc.9b05072.
15.
Li J, Thangaiyan R, Govindasamy K, et al. Anti-inflammatory and anti-apoptotic effect of zingiberene on isoproterenol-induced myocardial infarction in experimental animals. Hum Exp Toxicol. 2020; 26: 96032712097513. doi: 10.1177/0960327120975131.
16.
Chen H, Tang X, Liu T, et al. Zingiberene inhibits in vitro and in vivo human colon cancer cell growth via autophagy induction, suppression of PI3K/ AKT/mTOR Pathway and caspase 2 deactivation. JBUON. 2019; 24: 1470 –1475.
17.
Yeo SK, Ali AY, Hayward OA, et al. β-Bisabolene, a Sesquiterpene from the Essential Oil Extract of Opoponax (Commiphora guidottii), Exhibits Cytotoxicity in Breast Cancer Cell Lines. Phyther Res. 2016; 30: 418–425. doi: 10.1002/ptr.5543.
18.
Cui Y, Shi Y, Bao Y, et al. Zingerone attenuates diabetic nephropathy through inhibition of nicotinamide adenine dinucleotide phosphate oxidase 4. Biomed Pharmacother. 2018: 99: 422–430. doi: 10.1016/j.biopha.2018.01.051.
19.
Choi JS, Ryu J, Bae WY, et al. Zingerone suppresses tumor development through decreasing cyclin D1 expression and inducing mitotic arrest. Int J Mol Sci. 2018: 19(9): 2832. doi: 10.3390/ijms19092832.
20.
Han Q, Yuan Q, Meng X, et al. 6-Shogaol attenuates LPS-induced inflammation in BV2 microglia cells by activating PPAR-γ. Oncotarget 2017; 8: 42001–42006. doi: 10.18632/oncotarget.16719.
21.
Kim YG, Kim MO, Kim SH, et al. 6-Shogaol, an active ingredient of ginger, inhibits osteoclastogenesis and alveolar bone resorption in ligature-induced periodontitis in mice. J Periodontol. 2020; 91: 809–818. doi: 10.1002/JPER.19-0228.
22.
Hassan SMA, Hassan AH. The possibility of using Shogaol for treatment of ulcerative colitis. Iran J Basic Med Sci. 2018; 21: 943–949. doi: 10.22038/ijbms.2018.28616.6932.
23.
Lete I, Allué J. The effectiveness of ginger in the prevention of nausea and vomiting during pregnancy and chemotherapy. Integr Med Insights. 2016; 11: 11–17. doi: 10.4137/IMI.S36273.
24.
Pertz HH, Lehmann J, Roth-Ehrang R, et al. Effects of ginger constituents on the gastrointestinal tract: Role of cholinergic M3 and serotonergic 5-HT3 and 5-HT4 receptors. Planta Med. 2011; 77: 973–978. doi: 10.1055/s-0030-1270747.
25.
Abdel-Aziz H, Windeck T, Ploch M, et al. Mode of action of gingerols ans shogaols in 5-HT3 receptors: Binding studies, cation uptake by the receptor channel and contraction of isolated guinea-pig ileum. Eur J Pharmacol. 2006; 530(1–2): 136–43. doi: 10.1016/j.ejphar.2005.10.049.
26.
Giacosa A, Morazzoni P, Bombardelli E, et al. Can nausea and vomiting be treated with Ginger extract? Eur Rev Med Pharmacol Sci. 2015; 19: 1291–1296.
27.
Stanisiere J, Mousset PY, Lafay S. How safe is ginger rhizome for decreasing nausea and vomiting in women during early pregnancy? Foods 2018; 7(4): 50. doi: 10.3390/foods7040050.
28.
Pongrojpaw D, Somprasit C, Chanthasenanont A. A Randomized Comparison of Ginger and Dimenhydrinate in the Treatment of Nausea and Vomiting in Pregnancy. J Med Assoc Thai. 2007; 90(9): 1703–1709.
29.
Portnoi G, Chng LA, Karimi-Tabesh L, et al. Prospective comparative study of the safety and effectiveness of ginger for the treatment of nausea and vomiting in pregnancy. Am J Obstet Gynecol. 2003; 189: 1374–1377. doi: 10.1067/S0002-9378(03)00649-5.
30.
Viljoen E, Visser J, Koen N, et al. A systematic review and meta-analysis of the effect and safety of ginger in the treatment of pregnancy-associated nausea and vomiting. Nutr J. 2014; 13: 20. doi: 10.1186/1475-2891-13-20.
31.
Lavdaniti M, Tsitsis N. Investigation of Nausea and Vomiting in Cancer Patients Undergoing Chemotherapy. Health Psychol Res. 2014; 2(3): 1550. doi: 10.4081/hpr.2014.1550.
32.
Crichton M, Marshall S, Marx W, et al. Efficacy of Ginger (Zingiber officinale) in Ameliorating Chemotherapy-Induced Nausea and Vomiting and Chemotherapy-Related Outcomes: A Systematic Review Update and Meta-Analysis. J Acad Nutr Diet. 2019; 119: 2055–2068. doi: 10.1016/j.jand.2019.06.009.
33.
Ryan JL, Heckler CE, Roscoe JA, et al. Ginger (Zingiber officinale) reduces acute chemotherapy-induced nausea: A URCC CCOP study of 576 patients. Support Care Cancer 2019; 20(7): 1479–1489. doi: 10.1007/s00520-011-1236-3.
34.
Panahi Y, Saadat A, Sahebkar A, et al. Effect of ginger on acute and delayed chemotherapy-induced nausea and vomiting: A pilot, randomized, open-label clinical trial. Integr Cancer Ther. 2012; 11(3): 204 –211. doi: 10.1177/1534735411433201.
35.
Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017; 128: 40–50. doi: 10.1016/j.diabres.2017.03.024.
36.
Drągowski P, Czyżewska U, Cekała E, et al. Diabetes as a social and economic problem. Pol. Przegląd Nauk o Zdrowiu 2014; 2(39): 163–166.
38.
Zhu J, Chen H, Song Z, et al. Effects of Ginger (Zingiber officinale Roscoe) on Type 2 Diabetes Mellitus and Components of the Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Evid. Based Complement Alternat Med. 2018: 5692962. doi: 10.1155/2018/5692962.
39.
Khandouzi N, Shidfar F, Rajab A, et al. The effects of ginger on fasting blood sugar, hemoglobin A1c, apolipoprotein B, apolipoprotein A-I and malondialdehyde in type 2 diabetic patients. Iran J Pharm Res. 2015; 14: 131–140. doi: 10.22037/ijpr.2015.1632.
40.
Alshathly MR. Efficacy of Ginger (Zingiber officinale) in Ameliorating Streptozotocin-Induced Diabetic Liver Injury in Rats: Histological and Biochemical Studies. J Microsc Ultrastruct. 2019; 7: 91–101. doi:10.4103/JM AU.JM AU_16 _19.
41.
Shidfar F, Rajab A, Rahideh T, et al. The effect of ginger (Zingiber officinale) on glycemic markers in patients with type 2 diabetes. J Complement Integr Med. 2015; 12: 165–170. doi: 10.1515/jcim-2014-0021.
42.
Abdulrazaq NB, Cho MM, Win NN, et al. Beneficial effects of ginger (Zingiber officinale) on carbohydrate metabolism in streptozotocin-induced diabetic rats. Br J Nutr. 2012; 108: 1194–1201. doi: 10.1017/S0007114511006635.
43.
Arzati MM, Honarvar NM, Saedisomeolia A, et al. The effects of ginger on fasting blood sugar, hemoglobin A1c, and lipid profiles in patients with type 2 diabetes. Int J Endocrinol Metab. 2017; 15(4): e57927. doi: 10.5812/ijem.57927.
44.
Mozaffari-Khosravi H, Talaei B, Jalali BA, et al. The effect of ginger powder supplementation on insulin resistance and glycemic indices in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Complement Ther Med. 2014; 22(1): 9–16. doi: 10.1016/j.ctim.2013.12.017.
45.
Mann S, Beedie C, Jimenez A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: review, synthesis and recommendations. Sport Med. 2014; 44(2): 211–221. doi: 10.1007/s40279-013-0110-5.
46.
Li X, Guo J, Liang N, et al. 6-Gingerol regulates hepatic cholesterol metabolism by up-regulation of LDLR and cholesterol efflux-related genes in HepG2 Cells. Front Pharmacol. 2018; 9: 159. doi: 10.3389/fphar.2018.00159
47.
Pourmasoumi M, Hadi A, Rafie N, et al. The effect of ginger supplementation on lipid profile: A systematic review and meta-analysis of clinical trials. Phytomedicine. 2018; 43: 28–36. doi: 10.1016/j.phymed.2018.03.043.
48.
Saravanan G, Ponmurugan P, Deepa MA, et al. Anti-obesity action of gingerol: Effect on lipid profile, insulin, leptin, amylase and lipase in male obese rats induced by a high-fat diet. J Sci Food Agric. 2014; 94: 2972–2977. doi: 10.1002/jsfa.6642.
49.
Arablou T, Aryaeian N, Valizadeh M, et al. The effect of ginger consumption on glycemic status, lipid profile and some inflammatory markers in patients with type 2 diabetes mellitus. Int J Food Sci Nutr. 2014; 65: 515–520. doi: 10.3109/09637486.2014.880671.
50.
Sacitharan PK. Ageing and osteoarthritis. Subcell Biochem. 2019; 91: 123–159. doi: 10.10 07/978-981-13-3681-2 _ 6.
51.
Koszowska A, Nowak J, Hawranek R. Choroba zwyrodnieniowa stawów w kontekście nadwagi i otyłości. Forum Zaburzeń Metab. 2015; 6(2): 56–63.
52.
Szymański M, Korzeniowska K, Jabłecka A. Nerkowe działania niepożądane związane ze stosowaniem NLPZ. Geriatria. 2014; 8: 1–9.
53.
Arulselvan P, Fard MT, Tan WS, et al. Role of Antioxidants and Natural Products in Inflammation. Oxid Med Cell Longev. 2016; 2016: 5276130. doi: 10.1155/2016/5276130.
54.
Van Breemen RB, Tao Y, Li W. Cyclooxygenase-2 inhibitors in ginger (Zingiber officinale). Fitoterapia 2011; 82: 38–43. doi: 10.1016/j.fitote.2010.09.004.
55.
Funk JL, Frye JB, Oyarzo JN, et al. Anti-inflammatory effects of the essential oils of ginger (Zingiber officinale Roscoe) in experimental rheumatoid arthritis. PharmaNutrition. 2016; 4(3): 123–131. doi: 10.1016/j.phanu.2016.02.004.
56.
Aryaeian N, Shahram F, Mahmoudi M, et al. The effect of ginger supplementation on some immunity and inflammation intermediate genes expression in patients with active Rheumatoid Arthritis. Gene. 2019; 698: 179–185. doi: 10.1016/j.gene.2019.01.048.
57.
CFR-Code of Federal Regulations Title 21. Chapter I – Food and Drug Administration. Departent of Health and Human Services. Subchapter B – Food For Human Consumption (Continued). Part 182 – Substances Generally Recognized as Safe. https://www.accessdata.fda.gov... (access 16.12.2020).
58.
Moneret-Vautrin DA, Morisset M, Lemerdy P, et al. Food allergy and IgE sensitization caused by spices: CICBAA data (based on 589 cases of food allergy). Allerg Immunol. 2002; 34(4): 135–40.
59.
Toorenenbergen A, Dieges PH. Immunoglobulin E antibodies against coriander and other spices. J Allergy Clin Immunol. 1985; 76(3): 477–81. doi: 10.1016/0091-6749(85)90730-4.
60.
Lopez-De-Los-Santos P, Gonzales-de-Olano D, Madrigal-Burgaleta R, et al. Allergy to ginger with Cysteine proteinase GP-I as the relevant allergen. Ann Allergy Asthma Immunol. 2018; 121(5): 624–625. doi: 10.1016/j.anai.2018.07.013.61.
61.
Stäger J, Wüthrich B, Johansson SC. Spice allergy in celery-sensitive patients. Allergy 1991; 46(6): 475–8. doi: 10.1111/j.1398-9995.1991.tb04228.x.
Przetwarzamy dane osobowe zbierane podczas odwiedzania serwisu. Realizacja funkcji pozyskiwania informacji o użytkownikach i ich zachowaniu odbywa się poprzez dobrowolnie wprowadzone w formularzach informacje oraz zapisywanie w urządzeniach końcowych plików cookies (tzw. ciasteczka). Dane, w tym pliki cookies, wykorzystywane są w celu realizacji usług, zapewnienia wygodnego korzystania ze strony oraz w celu monitorowania ruchu zgodnie z Polityką prywatności. Dane są także zbierane i przetwarzane przez narzędzie Google Analytics (więcej).
Możesz zmienić ustawienia cookies w swojej przeglądarce. Ograniczenie stosowania plików cookies w konfiguracji przeglądarki może wpłynąć na niektóre funkcjonalności dostępne na stronie.
Możesz zmienić ustawienia cookies w swojej przeglądarce. Ograniczenie stosowania plików cookies w konfiguracji przeglądarki może wpłynąć na niektóre funkcjonalności dostępne na stronie.